Kinetin - Chemistry, Biology and Mechanism of Action
Introduction
Retinoids and hydroxy acids have successfully been used as active ingredients to improve the appearance of aging skin. However, both retinoids and hydroxy acids may be associated with skin irritation, stimulating a search for alternatives. Possible alternatives include kinetin (N6-furfuryladenine), zeatin, and pyratine-6. Kinetin and zeatin are members of a plant growth hormone family known as cytokinins, which have growth-promoting and antiaging effects in plants. Pyratine-6 (furfurylaminotetrahydropyranyladenine) is a synthetic analog of kinetin. The incorporation of these materials into cosmeceuticals is reviewed here.
Chemistry
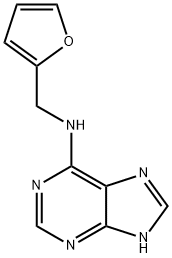
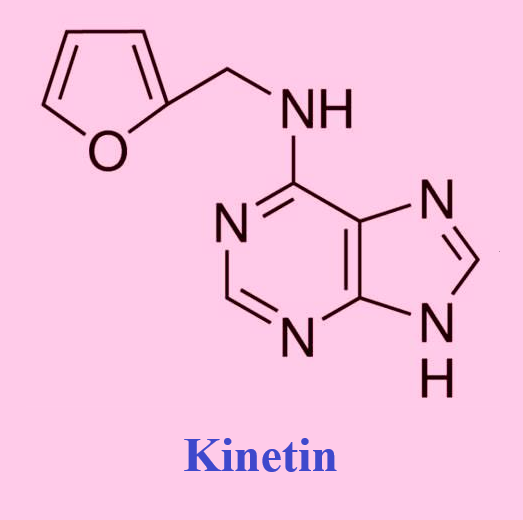
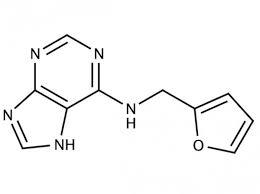
Kinetin was first isolated from autoclaved herring sperm DNA in 19551, and is a derivative of the nucleic acid purine base adenine. It is a naturally present base modification and is present in both plants and human cell extracts. The chemical structure of kinetin suggests that it can be formed from adenine and furfuryl (Figure 9.1). Furfuryl is formed after the primary oxidation of the deoxyribose moiety of DNA. It is not known how free kinetin is formed and if it is through the action of DNA repair enzymes that may remove this modified base from the DNA. Zeatin and pyrantine-6 are derived from adenine as well. Zeatin contains adenine with the addition of an hydroxy-methylbutyl group (Figure 9.2); pyratine-6 is similar to kinetin except for the addition of a tetrahydropyranyl group.
Biology
The first cytokinin identified and the most studied is kinetin. Cytokinins are plant growth substances that promote cell division and possibly cell differentiation. Plant-based studies are responsible for most of the data regarding the biological properties of kinetin. Kinetin stimulates tRNA synthesis and cell cycle progression9 in plants. Low levels of kinetin stimulate calcium influx through plant cell plasma membranes. Kinetin prevents yellowing and senescence of leaves and reduces the over ripening and degeneration of fruits.
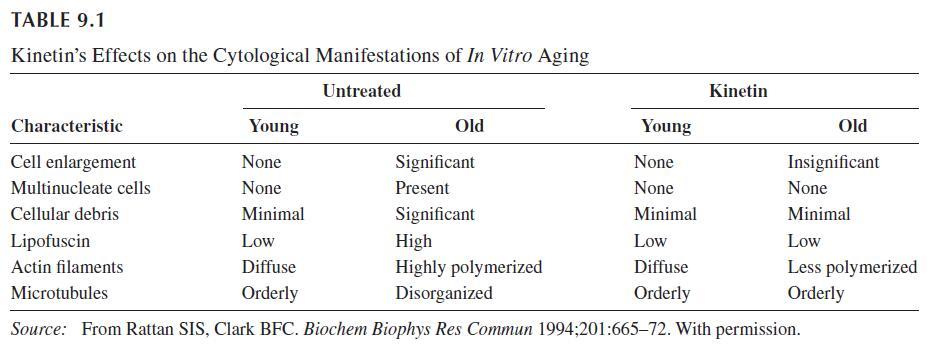 FIGURE 9.1 Chemical structure of adenine, furan, and kinetin.
FIGURE 9.1 Chemical structure of adenine, furan, and kinetin.
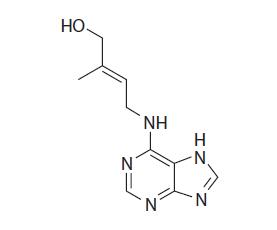
FIGURE 9.2 Chemical structure of zeatin.
A diet containing 20–50 ppm kinetin fed to fruit flies prolonged average and maximum life span by 65% and 35%, respectively. This was accompanied by a 55%–60% increase in the antioxidant enzyme catalase. Catalase is an enzyme that metabolizes hydrogen peroxide associated with cell toxicity. Kinetin has inhibitory activity on free radical formation of active platelets in vitro and thrombus formation in vivo.16 This warrants further study of kinetin to mitigate arterial thrombosis, and further studies are needed to assess whether kinetin has a role in the prevention and treatment of arterial thrombosis.
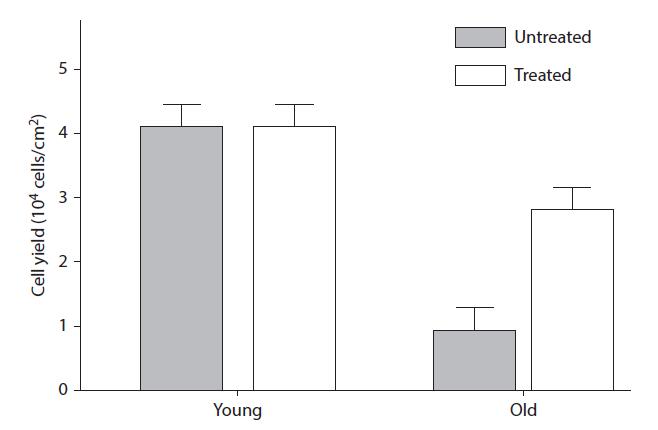
FIGURE 9.3 Cell yield in untreated and kinetin-treated young and old cells. (From Rattan SIS, Clark BFC. Biochem Biophys Res Commun 1994;201:665–72. With permission.)
A cytokinin nucleoside, N6-fufuryladenosine, has been shown to have antiproliferative and apoptogenic
activity against various human cancer cell lines, although similar activity has not been shown
with kinetin.
Mechanism of Action
Kinetin’s mechanism remains unclear. Kinetin may act directly as a signaling molecule, stimulating defense pathways such as DNA repair. Kinetin promotes calcium-induced differentiation of human keratinocytes, which becomes progressively delayed during aging.
Kinetin is implicated in several antioxidative roles. Kinetin may function as a natural antoxidant, preventing the formation of reactive oxygen species, or as a direct free radical scavenger. Oxygen radicals abstract hydrogen from the a-carbon of the amine bond in N6-furfuryladenine and these radicals undergo a faster dismutation reaction when kinetin is complexed with copper. Kinetin has been shown to protect against the formation of 8-oxo-2-deoxyguanosine, a marker of oxidative damage in DNA.
Kinetin has also been shown to protect against oxidative and glycoxidative protein damage generated in vitro by sugars and an iron/ascorbate system.
The significance of kinetin’s interaction with DNA or its antioxidant properties remains unknown. However, pluripotency may serve as a required prerequisite to act effectively as an agent of antiaging. Both in vitro and in vivo studies comparing the oxidative stress capacity of multiple antioxidants demonstrated that kinetin was not as potent as others such as tocopherol and ascorbic acid, although it was more potent than lipoic acid.
Conclusion
Kinetin (N6-furfuryladenine), a plant growth regulator, delays a range of cellular changes associated with the aging of human skin cells in vitro. In addition, kinetin has antioxidant properties. Clinical studies have demonstrated improvements in photodamaged skin. More active to vehicle comparison studies are needed.34 Studies suggest that kinetin is not associated with significant irritation and may serve as a potential alternative for individuals sensitive to other topical agents such as retinoids and hydroxy acids. Analogs of kinetin appear promising for topical use.
REFERENCES
1. Miller CO, Skoog F, Von Saltza MH et al. Kinetin, a cell division factor from deoxyribonucleic acid.
J Am Chem Soc 1955;77:1392.
2. Miller CO, Skoog F, Okumura FS et al. Isolation, structure, and synthesis of kinetin, a substance promoting
cell division. J Am Chem Soc 1956;78:1375–80.
3. Barciszewski J, Siboska GE, Pedersen BO et al. A mechanism for the in vivo formation of N6-furfuryladenine,
kinetin as a secondary oxidative damage product in DNA. FEBS Lett 1997;414:457–60.
4. Ramman N, Elumalai S. Presence of cytokinin in the root nodules of Casuarina equisetifolia. Ind J Exp
Biol 1996;34:577–9.
5. Ratti N, Janardhanan KK. Effect on growth of and cytokinin contents of pamlrosa (Cymbopogon martinii
var. motia) by Glomus inoculation. Ind J Exp Biol 1996;34:1126–8.
6. Barciszewski J, Siboska GE, Pedersen BO et al. Evidence for the presence of kinetin in DNA and cell
extracts. FEBS Lett 1996;393:197–200.
7. Kahn QA, Hadi SM. Effect of furfural on plasmid DNA. Biochem Mol Biol Int 1993;29:1153–60.
8. Guadino RJ. Cytokinin induction of RNA polymerase I transcription in Arabidopsis thaliana. J Biol
Chem 1997;272:6799–804.
9. Zhang K, Letham DS, John PC. Cytokinin controls the cell cycle at mitosis by stimulating the tyrosine
dephophorylation and activation of p34cdc-2-like H1 histone kinase. Planta 1996;200:2–12.
10. Shumaker KS, Gizinski MJ. Cytokinin stimulates dihydropyridine-sensitive calcium uptake in moss protoplasts.
Proc Natl Acad Sci USA 1993;90:10937–41.
You may like
Related articles And Qustion
Lastest Price from Kinetin manufacturers

US $0.00/KG2025-04-21
- CAS:
- 525-79-1
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 500kg/month

US $10.00/PCS2025-04-21
- CAS:
- 525-79-1
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 10 mt