Is vanillin polar?
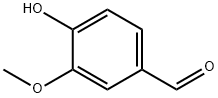
Vanillin is a phenolic aldehyde. Its functional groups include aldehyde, hydroxyl, and ether. It is the primary component of the extract of the vanilla bean. Synthetic vanillin is now used more often than natural vanilla extract as a flavoring in foods, beverages, and pharmaceuticals.

Polarity of vanillin
A polar molecule is one where some of the atoms are more electronegative, that is they attract electrons more than the other atoms.
Where C-C and C-H bonds are non polar but C-O and O-H bonds are polar. Due to the cumulative effect of the functional groups of the compound, vanillin is a polar molecule.
Uses
The largest use of vanillin is as a flavoring, usually in sweet foods. The ice cream and chocolate industries together comprise 75% of the market for vanillin as a flavoring, with smaller amounts being used in confections and baked goods.
Vanillin is also used in the fragrance industry, in perfumes, and to mask unpleasant odors or tastes in medicines, livestock fodder, and cleaning products. It is also used in the flavor industry, as a very important keynote for many different flavors, especially creamy profiles such as cream soda.
Related articles And Qustion
See also
Lastest Price from Vanillin manufacturers

US $0.00/kg2025-09-02
- CAS:
- 121-33-5
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $0.00/kg2025-07-18
- CAS:
- 121-33-5
- Min. Order:
- 1000kg
- Purity:
- 99.5%
- Supply Ability:
- 10000kg