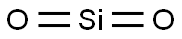Is SiO2 Polar or Nonpolar?
Dec 25,2023
Silicon dioxide (SiO₂), also known as silica, is a non-polar molecule.
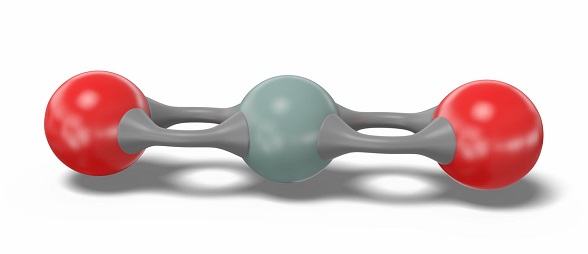
Each Si=O bond in the SiO2 molecule is polar due to an electronegativity difference between the bonded Si and O atoms. The electronegativity of the oxygen (O) atom is greater than the silicon (Si) atom. The O atoms strongly attract the shared electron pairs in each Si=O bond. The oxygen atoms are positioned on opposite sides of the silicon atom, resulting in a symmetric arrangement.
The molecule's geometry and symmetry lead to its non-polar nature. Thus, the net dipole moment comes out to be zero, and SiO2 is non-polar.
You may like
Related articles And Qustion
Application of Quartz
Mar 21, 2022
See also
Lastest Price from Quartz manufacturers
Quartz

US $10.00/ASSAYS2025-08-21
- CAS:
- 14808-60-7
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton
Quartz

US $999.00-666.00/kg2025-04-21
- CAS:
- 14808-60-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000