Is Selenium tetrachloride a polar molecule?
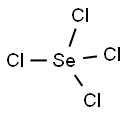
Selenium tetrachloride is an inorganic compound composed with the formula SeCl4. This compound exists as a yellow-to-white volatile solid. It is one of two commonly available selenium chlorides, the other example being selenium monochloride, Se2Cl2. SeCl4 is used in the synthesis of other selenium compounds.
In SeCl4, only one type of bond is formed: 'Se-Cl.'
The electronegativity of Se and Cl is 2.55 and 3.16, respectively. The difference comes out to be 0.61. Thus, the bonds are polar.
Polar bonds do not guarantee a polar molecule. The geometry and symmetry of the compound should be considered as well.
The capacity of strongly pulling the electron cloud of a covalent bond towards the atom is the measurement of the electronegativity of that atom. The electronegativity gives a direction of dipole moment. If these direction vectors are not canceled out of each other, a net dipole moment is generated.
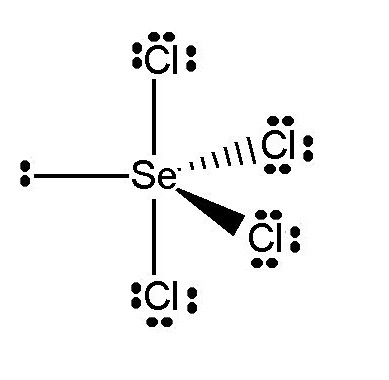
In the Selenium tetrachloride molecule, the Selenium atom has six outermost orbit electrons, whereas the Chlorine atom has seven outer shell electrons. The seCl4 molecule has a total of 34 outermost orbit electrons. Among these, eight electrons (4 pairs of electrons) make a bond in the molecule, and Selenium has two unshared electrons (one pair of electrons).
The geometry of the molecule should be trigonal bipyramidal. From VSEPR theory, we know that lone pair-bond pair repulsion is more significant than bond pair-bond pair repulsion. The molecule's shape becomes distorted tetrahedral for the nonbonding electron pair of the central atom Selenium.
The direction of dipole moments for electronegativity difference for the distorted shape can't neutralize each other. Selenium tetrachloride becomes a polar molecule.