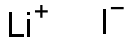Is lithium iodide ionic?
The rules of electronegativity differences:
1. If the electronegativity difference (usually called DEN) is less than 0.5, then the bond is nonpolar covalent.
2. If the DEN is between 0.5 and 1.6, the bond is considered polar covalent.
3. If the DEN is greater than 2.0, then the bond is ionic.
This leaves us with a problem - what about the gap between 1.6 and 2.0? Here we have rule #4:
4. If the DEN is between 1.6 and 2.0, and if a metal is involved (such as lithium), then the bond is considered ionic. If only nonmetals are involved, the bond is considered polar covalent.
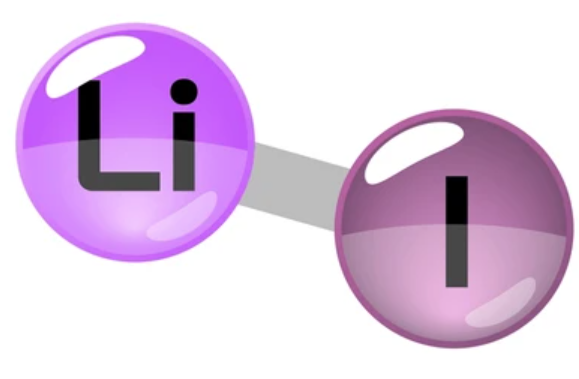
LiI has an electronegativity of 1.7 and is therefore considered ionic per Rule #4.
But you should also know that there is no clear strick line to determine between ionic and covalent bonds. Between 100% covalent bond and 100% ionic bond, there is a range of bonds partially ionic and covalent. Because Li+ is small and has high polarizing power, whereas I- is a voluminous anion, we should expect that even if LiI is ionic, it has a high proportion of covalent properties.
You may like
Lastest Price from Lithium iodide manufacturers

US $1.00/KG2025-04-29
- CAS:
- 10377-51-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1T

US $0.00-0.00/KG2025-04-15
- CAS:
- 10377-51-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg