Is DSIP Delta-Sleep-Inducing Peptide Safety?
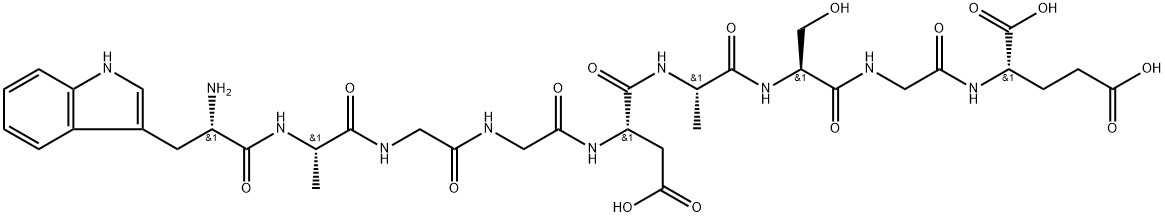
DSIP (delta-sleep-inducing-peptide), a nonapeptide (Trp-Ala-Gly-Gly-Asp-Ala-Ser-Gly-Glu: mol. wt 849), has been isolated from extracorporeal dialysate of cerebral sagittal venous blood from rabbits during electrical stimulation of the thalamus. The final isolation, amino acid analysis, sequence and synthesis, and the delta EEG (sleep)- --inducing properties of the synthetic compound were first reported in 1976-1978. The compound's sleep-promoting and related general physiological effects after intraventricular (brain), intravenous, and intraperitoneal application in different mammalian species have since been confirmed.

However, like any peptide, DSIP has a few potential side effects. These may include headaches, dizziness, and gastrointestinal issues like nausea or stomach pain. Moreover, the United States Food and Drug Administration (FDA) has not approved DSIP (Delta-Sleep-Inducing Peptide) for any therapeutic application. DSIP is a research peptide that is not currently approved as a medicine or treatment for any medical condition. It has not been subjected to the thorough testing and regulatory scrutiny required for FDA approval.
References
[1] D Schneider-Helmert, G A Schoenenberger. “The influence of synthetic DSIP (delta-sleep-inducing-peptide) on disturbed human sleep.” Experientia 37 9 (1981): 913–7.
You may like
See also
Lastest Price from DSIP manufacturers

US $10.00/box2025-12-08
- CAS:
- 62568-57-4
- Min. Order:
- 1box
- Purity:
- 99.99%
- Supply Ability:
- 9999

US $1.00/kg2025-11-06
- CAS:
- 62568-57-4
- Min. Order:
- 0.10000000149011612kg
- Purity:
- 99%
- Supply Ability:
- 200kg