Iron boride:Crystal structure,Properties,Uses
Iron boride(FeB) is a gray crystal, it is soluble in donor solvents such as alcohol ether and acetic acid but less soluble in hydrocarbons. The solid has a similar structure to that of FeCl3.
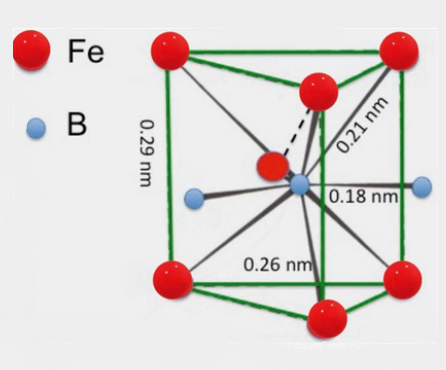
Crystal structure
Iron boride has a structure of Orthorhombic. Boron atoms have a slightly distorted mono-capped trigonal prismatic iron atom coordination and two boron atom neighbors. B-B single bond distance is 178 pm, Fe-B distance is 215–220 pm, and Fe-Fe distance is 240–272 pm. Each trigonal prism shares two rectangular faces with the nearby prisms, forming infinite prism columns.
Properties
Iron boride is a soft ferromagnetic compound that becomes paramagnetic above ~325 °C (617 °F).In the air, FeB powders begin to react with the ambient oxygen above 300 °C, though bulk FeB materials are expected to be stable in air to much higher temperatures. Iron boride is an extremely hard compound (15-22 GPa as measured by Vickers indentation), but is not sought after on bored steels because FeB layers are brittle and prone to spalling of the steel or iron.
Uses
Iron boride is used in the production of borated steels and borated stainless steels, deoxidizing agents, increase the hardness of steel, and alloying components of coatings applied to weld-facing electrodes. It is also used to improve abrasion resistance, wear resistance, and oxidation resistance.
Morover, it is used in oil and gas refinery, chemical extraction, automotive, agricultural, stamping, textile extrusion and injection molding industries.