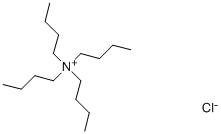
Introducton of Tetrabutylammonium chloride
General description
Tetrabutylammonium chloride is an organic chloride salt comprising of a tetrabutylammonium cation and chloride anion. It is a tetrabutylammonium salt and an organic chloride salt. Melting point: 83 ~ 86 ℃. Properties: colorless or light brown crystal. Deliquescent. Easily soluble in water, ethanol, chloroform and acetone, slightly soluble in benzene and ether. Irritating. Commodities also contain crystal water[1]. Tetrabutyl ammonium chloride is a chemical substance with the molecular formula of C16H36ClN.
Application and Pharmacology
A new protocol was developed for the site-selective C(sp3)–H chlorination of amides with tetrabutyl ammonium chloride as the chlorinating agent. The reaction features a tandem sequence that involves a (diacetoxyiodo)benzene-mediated and chloride anion-involved N–H chlorination followed by photoinitiated chlorine atom transfer. A wide variety of carboxamides and sulfonamides were chlorinated at the δ-position by using this method[3].
Figure 1 the application of Tetrabutylammonium chloride
A novel quaternary ammonium salt-tetrabutyl ammonium chloride(TBAC)is chosen to generate hydrate for cold storage The influence of solution concentration, cycle time, and borax as nucleation agent on the hydrates formation characteristics are studied Resultshowed that the solution with mass concentration of 40% has morestable phase transition processwhich is more suitable for air conditioning cold storage system. Addition of some nucleating agent such as borax in appropriate concentration to TBAC solution could decrease the phase transition temperature 2and the super cooling degree of crystallization could be decreased 2After cycle reaction was repeated for some times the super-coo ling degree is decreased 1.5℃,without any change of phase transition temperature The DSC test has shown that the tetrabutyl ammonium chloride hydrate takes phase transition under the temperature of 10 41℃. Based on the experimental study of tetrabutyl ammonium chloride hydrate (TBAC), a new cold storage working medium, the following conclusions are drawn[1].
(1) the effect of TBAC solution concentration on hydrate formation characteristics was experimentally studied. The experimental results show that the phase transition temperature of tetrabutylammonium chloride solution is affected by its solution concentration, and the phase transition temperature increases with the increase of concentration. The phase transition process of the solution with concentration of 40% is relatively stable, which is more suitable for the cold storage condition of air conditioning.
(2) adding proper concentration of nucleating agent borax in the experiment reduces the phase transition temperature of TBAC hydrate by 2 ℃, and reduces the undercooling of solution cooling crystallization by 2 ℃.
(3) after repeated hydration reactions, the undercooling of TBAC solution during hydrate formation decreased by 1.5 ℃, and the repeated reaction did not affect the phase transition temperature.
(4) DSC test shows that the phase transition temperature of TBAC hydrate is 10.41 ℃ and the latent heat of phase transition is 197.707 J/G-1, which verifies the feasibility of tetrabutylammonium chloride hydrate as a new cold storage working medium.
The experimental results show that TBAC hydrate can be used as a new type of cold storage working medium. Further research on TBAC hydrate will promote the practicability of quaternary ammonium hydrate cold storage working medium.
In order to develop an efficient synthesis method of enol silicone ether, the series reaction process of nucleophilic addition / brook rearrangement / elimination of nitroalkanes and silicon acetaldehyde esters was used to realize the synthesis of enol silicone ether under phase transfer conditions The results show that compared with the traditional synthesis method, the reaction conditions are mild, the operation is simple, the use of precious metals is avoided, the reaction yield is high and there are fewer by-products[2].
Safety and Storage
Easy to absorb moisture, sealed and dry. 97% ion pair chromatography special appearance white to light yellow crystalline solid white to light yellow crystalline solid infrared spectrum identification infrared spectrum method conforms to purity ≥ 97.0% (silver titration) ≥ 99.0% (silver titration) water solubility test water solubility.(BaiDu)
References
1.The cold storage characteristics of tetrabutylammonium chloride hydrate were studied.
2.Zhao Zhenguo, Zhu Chen, Zhou Yanan, et al. "Series reaction of nitromethane and silyl acetaldehyde catalyzed by tetrabutylammonium chloride", Journal of Huaibei Normal University (NATURAL SCIENCE EDITION), No. 01, 2022, pp. 25-30.
3.Zhu Y, Yu W. Photoinduced C(sp3)-H chlorination of amides with tetrabutyl ammonium chloride. Org Biomol Chem. 2021 Dec 1;19(46):10228-10232. doi: 10.1039/d1ob02081a. PMID: 34806744.
You may like
See also
Lastest Price from Tetrabutyl ammonium chloride manufacturers

US $1.00-2.10/KG2025-05-21
- CAS:
- 1112-67-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1KG 100KG 1MT

US $0.00/KG2025-04-21
- CAS:
- 1112-67-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 MT