Introduction to Triisopropylsilane: A Versatile Reagent in Organic Synthesis
Triisopropylsilane is a chemical compound that has garnered significant attention in the field of organic chemistry due to its versatile applications, particularly in the realm of organic synthesis. As a silicon-based hydride reagent, TRIISOPROPYLSILANE is widely used for various reduction reactions, playing a critical role in deprotection and radical-mediated processes. This article aims to provide a comprehensive overview of Triisopropylsilane, exploring its properties, key components, applications, and storage requirements.
Introduction
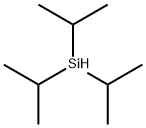
Triisopropylsilane is a trialkylsilane with the chemical formula C9H22Si. It is characterized by its three isopropyl groups attached to a central silicon atom. This structure imparts TRIISOPROPYLSILANE with unique physical and chemical properties, making it a valuable reagent in organic synthesis. The compound is a colorless liquid at room temperature, with a mild, distinct odor.
TRIISOPROPYLSILANE is frequently employed in laboratories and industrial settings due to its ability to act as a mild reducing agent. Its application ranges from the reduction of azides, nitro groups, and sulfoxides to the cleavage of silyl ethers. Moreover, TRIISOPROPYLSILANE is often used in conjunction with other reagents, enhancing its efficacy in complex synthetic pathways.

Figure 1 Characteristics of Triisopropylsilane
Properties
The unique properties of Triisopropylsilane stem from its molecular structure and silicon-hydrogen bond. Here are some of the key properties of TRIISOPROPYLSILANE:
Molecular Formula: C9H22Si
Molecular Weight: 158.36 g/mol
Boiling Point: Approximately 161°C (322°F)
Melting Point: -70°C (-94°F)
Density: 0.746 g/cm³ at 20°C
Refractive Index: 1.423-1.425 at 20°C
Flash Point: 37°C (98.6°F) – making it a relatively low flash point substance, requiring careful handling.
The silicon-hydrogen bond in TRIISOPROPYLSILANE is less reactive compared to other hydrosilanes, such as trimethylsilane. This reduced reactivity allows for selective reductions, which is highly beneficial in multi-step organic synthesis where control over reaction conditions is crucial.
Main Components
Triisopropylsilane is composed of a silicon atom bonded to three isopropyl groups and one hydrogen atom. The presence of these isopropyl groups increases the steric hindrance around the silicon center, providing TRIISOPROPYLSILANE with its distinct characteristics:
Silicon Atom: The silicon center is the key to the reactivity of TRIISOPROPYLSILANE, as it forms a bond with the hydrogen atom that can be utilized in reduction reactions.
Isopropyl Groups: The three isopropyl groups attached to the silicon provide steric bulk, which influences the selectivity of reactions involving TRIISOPROPYLSILANE. This bulkiness helps in avoiding over-reduction and enables selective reaction pathways.
Hydrogen Atom: The hydrogen bonded to silicon is the active component in reduction reactions. In radical processes, this hydrogen is often abstracted to generate a silyl radical, which plays a pivotal role in the subsequent reaction steps.
Applications
Triisopropylsilane's applications are vast and varied, primarily due to its ability to act as a reducing agent and its compatibility with various organic substrates. Below are some of the most common applications:
1. Reduction Reactions
TRIISOPROPYLSILANE is extensively used in the reduction of functional groups such as azides, nitro groups, and sulfoxides. It is particularly favored in cases where mild reaction conditions are required. For instance, TRIISOPROPYLSILANE is often employed in the reduction of azides to amines without affecting other sensitive functional groups in the molecule.
2. Deprotection of Silyl Ethers
In organic synthesis, protecting groups are frequently used to shield reactive sites in a molecule. Silyl ethers are a common protecting group for alcohol. TRIISOPROPYLSILANE can be used to selectively cleave silyl ethers, thereby deprotecting the alcohol group while leaving other functional groups intact. This selectivity is crucial in complex syntheses where multiple protecting groups might be present.
3. Radical-Mediated Reactions
TRIISOPROPYLSILANE is also used in radical chemistry, where it acts as a hydrogen atom donor. It is commonly employed in the reduction of radicals, helping to terminate radical chain reactions. This property is especially useful in the synthesis of complex organic molecules, where control over radical processes is essential.
4. As a Co-reagent
In many organic reactions, TRIISOPROPYLSILANE is used in conjunction with other reagents to enhance reaction efficiency. For example, it is often paired with palladium catalysts in cross-coupling reactions, where it aids in the reduction of intermediates and increases overall reaction yield.
Storage
Proper storage of Triisopropylsilane is essential to maintain its stability and prevent hazardous situations. Here are the key guidelines for storing TRIISOPROPYLSILANE:
Temperature: TRIISOPROPYLSILANE should be stored at a controlled room temperature, ideally between 15-25°C (59-77°F). It is important to avoid temperatures above its flash point, which could lead to flammability risks.
Containers: TRIISOPROPYLSILANE should be stored in tightly sealed containers to prevent exposure to air and moisture. The compound is stable under inert conditions, but contact with moisture can lead to hydrolysis, which can degrade the reagent.
Ventilation: Due to its low flash point, TRIISOPROPYLSILANE should be stored in a well-ventilated area, away from sources of ignition such as open flames or electrical sparks. This precaution minimizes the risk of fire hazards.
Incompatibilities: TRIISOPROPYLSILANE should be kept away from strong oxidizing agents, acids, and bases, as these substances can react with the silane and lead to hazardous conditions.
Conclusion
Triisopropylsilane is a versatile and indispensable reagent in the field of organic synthesis. Its unique properties, stemming from the steric hindrance of the isopropyl groups and the reactivity of the silicon-hydrogen bond, make it an essential tool for chemists. TRIISOPROPYLSILANE is widely used in reduction reactions, deprotection of silyl ethers, and radical-mediated processes, highlighting its broad applicability in complex synthetic pathways.
Given its reactive nature, proper storage and handling are crucial to ensure safety and maintain the reagent's efficacy. As research in organic synthesis continues to evolve, Triisopropylsilane will likely remain a cornerstone reagent, facilitating the development of new synthetic methodologies and the production of complex organic molecules.
[1] Ellsworth B A, Doyle A G, Patel M, et al. C-Arylglucoside synthesis: triisopropylsilane as a selective reagent for the reduction of an anomeric C-phenyl ketal[J]. Tetrahedron: Asymmetry, 2003, 14(20): 3243-3247.
[2] Ste. Marie E J, Hondal R J. Reduction of cysteine‐S‐protecting groups by triisopropylsilane[J]. Journal of Peptide Science, 2018, 24(11): e3130.
References:
[1] B. ELLSWORTH. C-Arylglucoside synthesis: triisopropylsilane as a selective reagent for the reduction of an anomeric C-phenyl ketal[J]. Tetrahedron, asymmetry, 2003, 30 1: 1311-1332. DOI:10.1016/J.TETASY.2003.08.007.[2] EMMA J. STE.MARIE R J H. Reduction of cysteine-S-protecting groups by triisopropylsilane[J]. Journal of Peptide Science, 2018, 24 11. DOI:10.1002/psc.3130.
Lastest Price from Triisopropylsilane manufacturers

US $0.00/KG2025-04-21
- CAS:
- 6485-79-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500kg/month

US $1000.00-400.00/ton2025-04-21
- CAS:
- 6485-79-6
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 5000