Dexamethasone 21-Phosphate Disodium Salt: A Promising Nebulised Therapy for Managing Severe Equine Asthma
General Description
Dexamethasone 21-phosphate disodium salt has emerged as a treatment for severe equine asthma due to its localized anti-inflammatory effects when nebulised. A study comparing nebulised and oral administration revealed that while both forms decreased serum cortisol levels, lung function improvements were limited, with nebulisation failing to significantly reduce pulmonary resistance. Moreover, the compound demonstrated low systemic bioavailability (4.3%) and minimal systemic side effects, indicating its effectiveness in targeting respiratory conditions. Overall, while nebulised dexamethasone 21-phosphate disodium salt offers a favorable tolerability profile, further research is needed to assess its optimal dosing and delivery strategies.

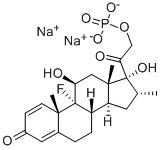
Figure 1. Dexamethasone 21-phosphate disodium salt
Applications in Equine Asthma Treatment
Introduction to Dexamethasone 21-Phosphate Disodium Salt in Equine Asthma Treatment
Dexamethasone 21-phosphate disodium salt has emerged as a potential treatment option for severe asthmatic horses, primarily due to its anti-inflammatory properties and effectiveness in addressing equine asthma symptoms. Inhaled corticosteroids, including dexamethasone 21-phosphate disodium salt, are recognized for their ability to reduce airway inflammation and improve pulmonary function, thereby enhancing the quality of life for affected horses. The use of this compound, particularly via nebulisation, offers a targeted approach aimed at minimizing systemic side effects while delivering the medication directly to the lungs, where it is needed most. 1
Efficacy of Nebulised Dexamethasone 21-Phosphate Disodium Salt
In a recent study focusing on severe asthmatic horses, the efficacy of dexamethasone 21-phosphate disodium salt administered through nebulisation was put to the test against oral administration. A total of twelve horses diagnosed with severe asthma were subjected to a controlled trial where they received either nebulised or orally administered dexamethasone 21-phosphate disodium salt over a week. The study aimed to assess improvements in lung function, marked by measures such as pulmonary resistance and elastance. Notably, the nebulised form was hypothesized to yield better outcomes than the oral alternative, as it could provide more localized action in the airways and potentially greater therapeutic benefit. 1
Observations and Conclusion
The results indicated that while serum cortisol levels decreased across the board—a sign of systemic corticosteroid effects—lung function improvements were limited. Specifically, although the oral group showed significant reductions in pulmonary resistance, the nebulised dexamethasone 21-phosphate disodium salt did not lead to notable enhancements in lung function. Residual bronchospasm was observed in all subjects at the study's conclusion, suggesting that the nebulisation of dexamethasone 21-phosphate disodium salt at the tested dose may not be a viable therapeutic strategy for severe equine asthma. Thus, this study calls into question the utility of nebulised dexamethasone 21-phosphate disodium salt as a first-line treatment, highlighting the need for further investigation into optimal dosages and delivery mechanisms. 1
Bioavailability and Tolerability
Bioavailability
Dexamethasone 21-phosphate disodium salt, when delivered via nebulisation, exhibits minimal systemic bioavailability. Research conducted on adult horses revealed an average maximum plasma concentration of this compound at 0.774 ng/mL, resulting in an overall systemic bioavailability of only 4.3%. This low level of bioavailability signifies that a large proportion of the administered dose remains concentrated within the respiratory tract. Such localized delivery is advantageous because it enhances the therapeutic effects of dexamethasone 21-phosphate disodium salt directly in the lungs while simultaneously minimizing systemic exposure and potential side effects associated with corticosteroid use. Consequently, nebulised dexamethasone 21-phosphate disodium salt is particularly effective for respiratory conditions, allowing for targeted treatment without significant systemic impact. 2
Tolerability
The tolerability profile of dexamethasone 21-phosphate disodium salt, when administered through nebulisation, has shown favorable outcomes. In the same study assessing bioavailability, there were no significant increases in airway inflammation, as indicated by stable bronchoalveolar lavage fluid profiles. Furthermore, nebulised administration did not result in the suppression of the hypothalamic-pituitary-adrenal axis, which is often a concern with systemic corticosteroid treatments. Unlike intravenous administration that significantly reduced serum cortisol levels, nebulised dexamethasone 21-phosphate disodium salt maintains normal systemic hormone levels. These results provide compelling evidence that dexamethasone 21-phosphate disodium salt is well-tolerated in healthy adult horses, making it a safe and effective option for managing respiratory conditions without compromising overall health. 2
References:
[1] A D HASPEL. Bioavailability and tolerability of nebulised dexamethasone sodium phosphate in adult horses.[J]. Equine Veterinary Journal, 2018, 50 1. DOI:10.1111/evj.12724.[2] S. MAINGUY-SEERS. Nebulisation of dexamethasone sodium phosphate for the treatment of severe asthmatic horses[J]. Equine Veterinary Journal, 2019, 51 5: 561-707. DOI:10.1111/evj.13091.
See also
Lastest Price from Dexamethasone 21-phosphate disodium salt manufacturers

US $5.00-0.50/KG2025-06-13
- CAS:
- 2392-39-4
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg

US $5.00-0.50/KG2025-05-30
- CAS:
- 2392-39-4
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS