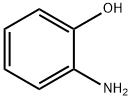
Industrial Synthesis of 2-Aminophenol
O-aminophenol, also known as 2-aminophenol, ortho-aminophenol (OPA), 1-amino-2-hydroxybenzene, is a white or light gray crystalline powder. 2-Aminophenol is a typical aromatic amphoteric compound and an important chemical intermediate, which is widely used in the fields of dyes, medicine, printing and biology. According to reports in the literature, o-aminophenol may cause allergic dermatitis in humans and may also cause methemoglobinemia in humans.
Industrial Synthesis
The main preparation processes of 2-aminophenol include iron powder reduction, hydrogenation reduction, nitration re-reduction and sodium sulfide reduction. Its traditional synthesis method is prepared by hydrolyzing o-nitrochlorobenzene as raw material and reducing iron powder. During the reduction process, the pollution of the three wastes is serious, and the product is packaged in the iron sludge, which makes the product yield not high. Ortho-nitrophenol can also be used as raw material ethanol as a solvent to prepare o-aminophenol. Because the solubility of o-nitrophenol (ONP) in water is too small during the reduction process, the organic solvent ethanol is mostly used in this process. However, because o-aminophenol (OAP) has a high solubility in organic solvents and is very unstable, the yield has not been high, and the cost of ethanol is also high, which makes the cost of this process slightly higher. However, with sodium sulfide reduction, the yield of the obtained product is very low, and because the wastewater contains a large amount of chloride ions, it also causes great pollution to the environment.
CN201310710958.5 provides a method for preparing o-aminophenol. Firstly, o-nitrochlorobenzene is used as a raw material, and sodium hydroxide solution is added for hydrolysis; then sodium hydrosulfide is added for reduction reaction to obtain sodium o-aminophenol, and then sulfuric acid solution is added Carry out acidification to obtain hydrogen sulfide gas, and pass the hydrogen sulfide absorption tower to obtain sodium hydrosulfide and then put it into production, and the sulfate in the solution can react with the sodium ions in the sodium nitrophenolate to obtain sodium sulfate, and then reduce the temperature to obtain sulfuric acid Sodium crystals; the acidified solution is filtered and then added to the alkaline solution and neutralized to obtain the final o-aminophenol. The waste water obtained after production contains free chloride ions, and limestone can be added to obtain calcium chloride precipitation; after the precipitation is removed, the industrial production water is obtained by decolorization of activated carbon and four-effect evaporation and put into production to achieve the purpose of recycling.
Related articles And Qustion
Lastest Price from 2-Aminophenol manufacturers

US $10.00/KG2025-04-21
- CAS:
- 95-55-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $56.00/kg2025-02-08
- CAS:
- 95-55-6
- Min. Order:
- 1kg
- Purity:
- 0.98
- Supply Ability:
- 100kg