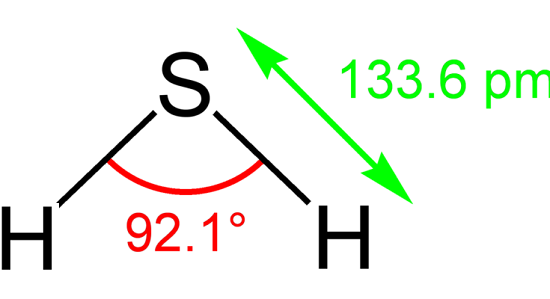
Hydrogen Sulfide-Hazard and Toxicity
Description
Hydrogen Sulfide is a colorless, very poisonous, flammable gas with the characteristic foul odor of rotten eggs. It often results from the bacterial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers where anaerobic digestion can take place. It also occurs in volcanic gases, “natural gas”, and some well waters. Hydrogen sulfide has numerous names, some of which are archaic.

Toxicity Data
LC50 inhal (rat) 444 ppm (580 mg/m3)
LCLO inhal (human) 800 ppm (1110 mg/m3; 5 min)
PEL (OSHA) 20 ppm (ceiling) (28 mg/m3)
TLV-TWA (ACGIH) 10 ppm (14 mg/m3)
STEL (ACGIH) 15 ppm (21 mg/m3)
Major Hazards
Moderately toxic gas; inhalation of large concentrations can cause unconsciousness, respiratory paralysis, and death; highly flammable. Exposure to very high concentrations causes immediate death. Also death or permanent injury may occur after very short exposure to small quantities. It acts directly upon the nervous system resulting in paralysis of respiratory centers.
Toxicity
The acute toxicity of hydrogen sulfide by inhalation is moderate. A 5-min exposure to 800 ppm has resulted in death. Inhalation of 1000 to 2000 ppm may cause coma after a single breath. Exposure to lower concentrations may cause headache, dizziness, and upset stomach. Low concentrations of H2S (20 to 150 ppm) can cause eye irritation, which may be delayed in onset. Although the odor of hydrogen sulfide is detectable at very low concentrations, it rapidly causes olfactory fatigue at higher levels, and therefore is not considered to have adequate warning properties. Hydrogen sulfide has not been shown to be carcinogenic or to have reproductive or developmental effects in humans.
Flammability and Explosibility
Hydrogen sulfide is flammable in air in the range of 4.3 to 45.5% (NFPA rating = 4). Combustion products (sulfur oxides) are also toxic by inhalation. In the event of a hydrogen sulfide fire, stop the flow of gas if possible without risk of harmful exposure and let the fire burn itself out.
Reactivity and Incompatibility
Hydrogen sulfide is incompatible with strong oxidizers. It will attack many metals, forming sulfides. Liquid hydrogen sulfide will attack some forms of plastics, rubber, and coatings. H2S reacts violently with a variety of metal oxides, including the oxides of chromium, mercury, silver, lead, nickel, and iron.
Storage and Handling
In particular, cylinders of hydrogen sulfide should be stored and used in a continuously ventilated gas cabinet or fume hood. Local fire codes should be reviewed for limitations on quantity and storage requirements.
Accidents
In the event of a release of hydrogen sulfide, the area should be evacuated immediately. Use appropriate respiratory protection to rescue an affected individual. Remove exposed individual to an uncontaminated area, and seek immediate emergency help. Keep victim warm, quiet, and at rest; provide assisted respiration if breathing has stopped. In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention.
To respond to a release, use appropriate protective equipment and clothing. Positive pressure air-supplied respiratory protection is required. Close cylinder valve and ventilate area. Remove cylinder to a fume hood or remote area if it cannot be shut off.
Disposal
Excess hydrogen sulfide should be returned to the manufacturer, according to your institution's waste disposal guidelines.