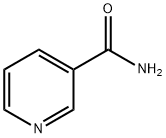
How to synthesize Nicotinamide?
Description
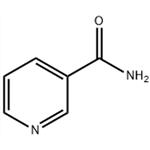
Nicotinamide (NAM) is a dietary source of nicotinamide adenine dinucleotide (NAD+). Gram doses of NAM have long been used to treat diverse disease conditions, such as inflammatory diseases and dementia, in humans and animal models[1]. Nicotinamide is divided into pharmaceutical-grade and feed-grade products. Pharmaceutical-grade products have a pH range of 5.5-7.5, according to the current version of the Chinese Pharmacopoeia. High-grade nicotinamide generally requires a pH value between 6.5 and 7.5; feed-grade cigarettes' Amide pH range is 5.0-6.0.
Synthesis method
Currently, cyanopyridine can be converted into amides through chemical and biological means. Japanese patent JP93-206579 and European patent EP85-306670 both disclose the use of modified Raney nickel catalysts for the reaction of converting cyanopyridine into amides. WO90/09988A1 discloses the use of alkali metal borates in converting cyanopyridine into amides. U.S. Patent US2,471,518, U.S. Patent US4,721,709, and German patent application DE2,517,054 all disclose the hydrolysis of 3-cyanopyridine in sodium hydroxide. However, the above existing methods have some shortcomings: although the conversion rate of 3-cyanopyridine is very high, the selectivity is very poor, and a part of the by-product nicotinic acid will be produced during the reaction, so the pH value of the nicotinamide product obtained is not high. , it is challenging to meet the requirements.
To this end, Hua et al. published a method for preparing nicotinamine. This method does not introduce auxiliary reagents, is economical, has relatively mild reaction conditions, is easy to separate the product, has high purity, high yield, and little pollution, and is conducive to industrial production. The steps are to dissolve 3-cyanopyridine in alcohol, add water and a catalyst to perform a hydrolysis reaction and obtain nicotinamide through post-processing of the reaction product.
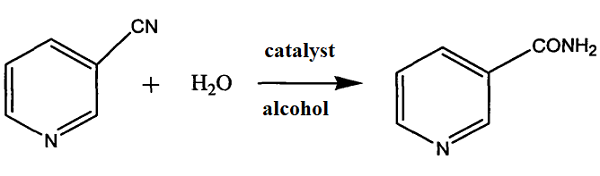
The specific steps are: In a 1000ml four-necked flask, add 20g manganese dioxide (0.23mol), 100g solid 3-cyanopyridine (0.96mol), and 400g ethanol with a mass concentration of 95% (including 380g ethanol, 8.25 mol; water 20g, 1.1lmol). Stir and raise the temperature to 90°C, then keep the temperature at about 90°C. End the reaction after 6 hours. After the reaction, the reaction product is sampled and detected by HPLC. The reaction product was then rotary evaporated to dryness and then taken out. After vacuum drying at 60°C for 10 hours, 116.7g of nicotinamide was obtained. The molar yield of nicotinamide was 99.49%, and the pH value was 7.0.
References
[1] Hwang, E. “Pharmacological Nicotinamide: Mechanisms Centered Around SIRT1 Activity.” 1900. 0.
[2] CN101851194B - Method for preparing nicotinamide - Google Patents.
You may like
Related articles And Qustion
See also
Lastest Price from Nicotinamide manufacturers
US $0.00/Kg2025-10-13
- CAS:
- 98-92-0
- Min. Order:
- 1Kg
- Purity:
- 98%
- Supply Ability:
- 20Ton

US $0.00/KG2025-08-26
- CAS:
- 98-92-0
- Min. Order:
- 1KG
- Purity:
- 99%MIN
- Supply Ability:
- 20tons