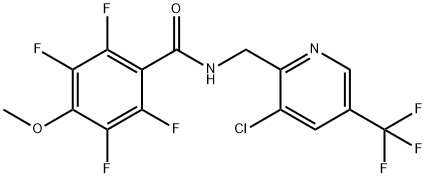
How to synthesize Fluopimomide?
Description
Fluopimomide ( FM; Kanuozi, Sino-Agri Union, Jinan, Shandong, China), registered in 2015, was first used to control oomycete diseases. It is not the first fungicide evaluated for managing plant-parasitic nematodes [1].
Fluopyram, a succinate dehydrogenase inhibitor fungicide, is also an excellent nematicide. Grabau et al. (2021) showed that in-furrow application of fluopyram at 236 g ha−1 effectively managed Rotylenchulus reniformis on cotton. FM has a similar chemical structure to fluopyram. It causes the redistribution of spectrin-like proteins, which are responsible for maintaining the membrane stability of Ascomycetes and Oomycetes, from the membrane to the cytoplasm. Fluopimomide was derived from the Oomycetes-selective fungicide fluopicolide and shares the same 3-chloro-5-(trifluoromethyl)-2- pyridinemethanamine portion. However, compared to fluopicolide, fluopimomide has a broader fungicidal spectrum. Besides Oomycetes pathogens such as Pseudoperonospora cubensis, it can also control other diseases such as tomato gray mold caused by Botrytis cinerea. Furthermore, it was recently shown that fluopimomide also expresses strong activity against Meloidogyne incognita (Southern root-knot nematode).
![Benzamide, N-[[3-chloro-5-(trifluoromethyl)-2-pyridinyl]methyl]-2,3,5,6-tetrafluoro-4-methoxy- Article illustration](/NewsImg/2024-02-18/6384384680702761508501926.png)
Synthesis method
Two different syntheses of fluopimomide (XII) start from the pentafluorinated benzoate 70, which can be obtained by esterification of pentaflurobenzoic acid or by Grignard reaction of bromopentafluorobenzene with ethyl chloroformate[2]. Introducing the required methoxy group into the 4-position of 70 with methoxytrimethylsilane in the presence of a tricoordinated gold(I) chloride complex leads to 71. Ester saponification under primary conditions and transformation of the resulting benzoic acid 72 with thionyl chloride yields 2,3,5,6-tetrafluoro-4-methoxybenzoyl chloride (73), which undergoes amidation with 3-chloro-5-(trifluoromethyl)-2-pyridinemethanamine to yield fluopimomide (XII). In an alternative route, the order of the described steps is reversed. The starting material ethyl 2,3,4,5,6-pentafluorobenzoate (70) can also be converted into the amide 69 by ester cleavage, acid chlorination, and amidation. The selective introduction of fluopimomide's methoxy function is possible in high yields with sodium methoxide in methanol.
![Benzamide, N-[[3-chloro-5-(trifluoromethyl)-2-pyridinyl]methyl]-2,3,5,6-tetrafluoro-4-methoxy- Article illustration](/NewsImg/2024-02-18/6384384689957645757561896.png)
Toxicity
In vitro, fluopimomide had high toxicity to second-stage juveniles (J2s) and eggs of M. incognita with median lethal concentrations (LC50) of 23.4 and 9.5 mg L−1 after 48 h exposure, respectively[3]. Under greenhouse conditions, fluopimomide at 250, 500, and 1000 g ha−1 was effective in reducing root galling at 30 and 60 days after treatment (DAT), with the value of the galling index being reduced by 19.3%–23.8% and 35.7%–52.7%, respectively, and increasing plant height, stem diameter, and above-ground biomass. Fluopimomide at all rates significantly reduced populations of M. incognita in the soil by 26.2–46.5% and 65.6%–82.1% at 30 and 60 DAT, respectively, compared with the untreated control. Moreover, there were no significant differences in the reduction of M. incognita J2s by fluopimomide at 1000 g ha−1 compared to fluopyram or abamectin.
References
[1] Weiping Zhang. “Management of Meloidogyne incognita on Cucumber with a New Nonfumigant Nematicide Fluopimomide.” Plant disease 106 1 (2022): 151–155.
[2] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.
[3] Jingjing Li . “Evaluating a new non-fumigant nematicide fluopimomide for management of southern root-knot nematodes in tomato.” Crop Protection 129 (2020): Article 105040.