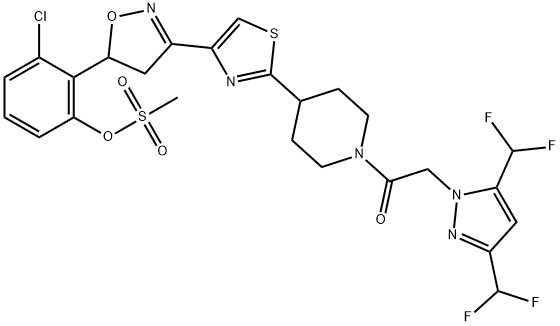
The PTI fungicide- Fluoxapiprolin
Description
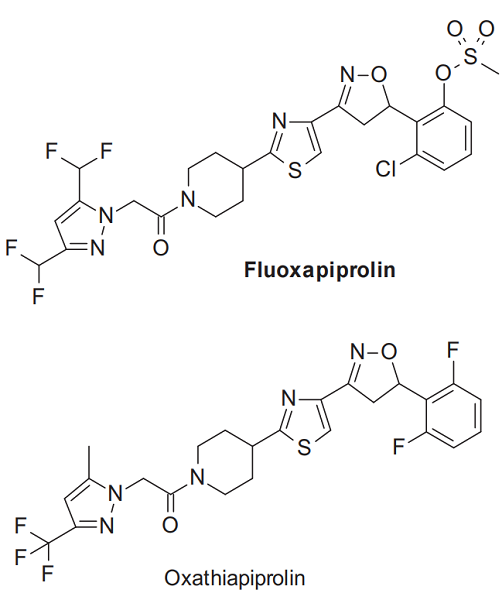
Fluoxapiprolin (development code BCS-CS55621), 2-{3-[2-(1-{[3,5-bis(difluoromethyl)-1H-pyrazol-1-yl]acetyl}-4-piperidinyl)-1,3-thiazol-4-yl]-4,5-dihydro-1,2-oxazol-5-yl}-3-chlorophenyl methanesulfonate, is an another PTI fungicide discovered and developed by Bayer Crop Science in 2012. Its molecular structure is highly similar to that of oxathiapiprolin, and the molecules of both are arranged in sequence with four heterocycles: pyrazole, piperidine, thiazole, and isoxazoline; at the same time, a benzene ring is also connected to the 5-position of the isoxazoline ring. Fluoxapiprolin is a racemate, and its R and S forms have almost the same biological activity. It has not been registered in any country, but some preregistration trials have been completed in China. It is highly effective against late blight and downy mildew diseases and, based on its close structural similarity to oxathiapiprolin, seems to share the same mode of action of oxysterol binding protein inhibition[1–2].
Synthesis method
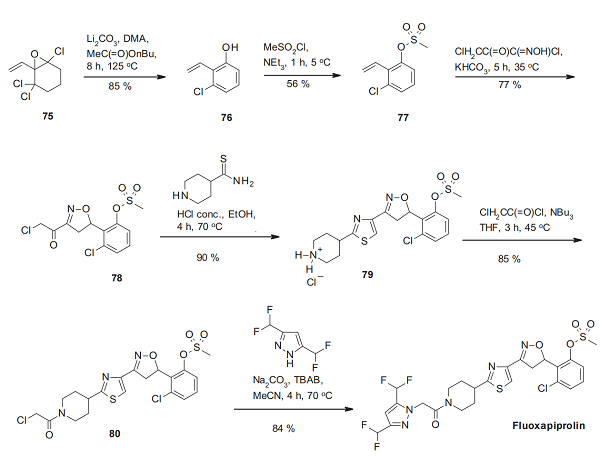
The synthesis of fluoxapiprolin starts from the trichlorinated and vinylated cyclohexene oxide 75, which delivers the o,o’-disubstituted styrene 76 by thermal aromatization. After sulfonylation to the corresponding mesylate 77, the conversion of the C–C double bond with chloroacetylhydroximinoyl chloride, which can be obtained by nitrosation of 4-chloroacetoacetate, delivers the α -chloroketosubstituted isoxazoline derivative 78. Its condensation with piperidine- 4-carbothioamide adds two further rings, piperidine and a thiazole, resulting in the piperidinium salt 79, which is subsequently transformed with chloroacetyl chloride into the chloroacetamide derivative 80. In the final step, nucleophilic substitution of this chlorine with 3,5-bis (difluoromethyl)pyrazole delivers fluoxapiprolin[3].
References
[1] Jianqiang Miao. “Activity and Resistance-Related Point Mutations in Target Protein PcORP1 of Fluoxapiprolin in Phytophthora capsici.” Journal of Agricultural and Food Chemistry 69 13 (2021): 3827–3835.
[2] Chengcheng Li and Xili Liu. “Resistance Risk and Novel Resistance-Related Point Mutations in Target Protein PiORP1 of Fluoxapiprolin in Phytophthora infestans.” Journal of Agricultural and Food Chemistry 70 16 (2022): 4881–4888.
[3] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.