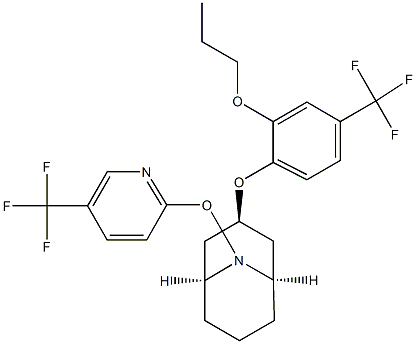
How to synthesize Acynonapyr?
Description
Nippon Soda announced Acynonapyr as a new acaricide 2017, developed under the code NA-89. It is effective against spider mites, such as Tetranychus urticae (two-spotted spider mite) and Panonychus ulmi (European red mite), in vegetables, tea, and citrus fruits. Recently, it has been reported that acynonapyr acts on glutamate receptors and disrupts neurotransmission.
Synthesis method
Method 1
The synthesis of acynonapyr starts with the construction of its azabicyclo[3.3.1]nonane core, which is achieved by the Robinson-Schoppf reaction of 1,3-acetone dicarboxylic acid (178), glutaraldehyde, and benzylamine. The resulting bridged bicyclic 179 is converted into 180 by ketone reduction and transforming the newly formed alcohol with 1-fluoro-2-propoxy-4-(trifluoromethyl)benzene. The reductive removal of the benzyl group and reaction with acrylonitrile leads to the intermediate 182. Under oxidative conditions, the ring nitrogen is oxygenated to 183, leading to the loss of propionitrile to the N-hydroxy derivative 184. Finally, the conversion of this hydroxy group with 2-chloro- 5-(trifluoromethyl)pyridine delivers acynonapyr[1].
![(1R,3r,5S)-3-[2-propoxy-4-(trifluoromethyl)phenoxy]-9-{[5-(trifluoromethyl)pyridin-2-yl]oxy}-9-azabicyclo[3.3.1]nonane Article illustration](/NewsImg/2024-02-26/6384455002593508228848935.png)
Method 2
The synthetic route to acynonapyr via N-hydroxylation of an NH-free azabicycle is shown below. Bicyclic ketone 2 was obtained by the Mannich reaction using glutaraldehyde, 3-oxopentanedioic acid, and benzylamine, and the subsequent ketone reduction gave 3-endo-ol 3 stereoselectively. O-arylated azabicycle 5 was obtained by coupling 3 with fluorobenzene 4, and debenzylation of 5 gave NH-free azabicycle 6. Cyanoethylamine 7 was obtained by reacting 6 with acrylonitrile, and oxidation of 7 gave hydroxylamine 8. Finally, coupling of 8 with chloropyridine 9 provided Acynonapyr.
![(1R,3r,5S)-3-[2-propoxy-4-(trifluoromethyl)phenoxy]-9-{[5-(trifluoromethyl)pyridin-2-yl]oxy}-9-azabicyclo[3.3.1]nonane Article illustration](/NewsImg/2024-02-26/6384455007484580056440677.png)
Method 3
Another synthetic route to acynonapyr via oxidation of azabicycle 10 is shown below. To a solution of 6, ·HCl salt (15.0 g, 39.6 mmol) in CH3CN (120 mL) was added chloropyridine 9 (21.50 g, 0.118 mol) and K2CO3 (21.9 g, 0.16 mol). The mixture was then heated under reflux and stirred overnight. After cooling, it was worked up as in procedure A. The residue was purified by silica gel chromatography (hexane/AcOEt, 98/2) to give azabicycle 10 (2.43 g, 13%): viscous oil.
![(1R,3r,5S)-3-[2-propoxy-4-(trifluoromethyl)phenoxy]-9-{[5-(trifluoromethyl)pyridin-2-yl]oxy}-9-azabicyclo[3.3.1]nonane Article illustration](/NewsImg/2024-02-26/6384455012082252833993334.png)
To a solution of azabicycle, 10 (1.00 g, 2 mmol) in CH2Cl2 (10 mL) was added m-CPBA (ca.65% purity, 0.81 g, 3 mmol) at room temperature. The mixture was then stirred overnight and diluted with AcOEt. The organic phase was washed with saturated Na2S2O4 aq., NaHCO3 aq., and water and dried over MgSO4. The solvent was removed under reduced pressure, and the residue was purified by silica gel chromatography (hexane/AcOEt, 98/2 → 95/5) to give acynonapyr (0.37 g, 37%)[2].
References
[1] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.
[2] Hamamoto, Isami et al. “Discovery of a novel acaricide, acynonapyr.” Journal of Pesticide Science 33 1 (2023): 0.