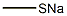How to synthesis Sodium thiomethoxide
Introduction
Sodium methyl mercaptide (Sodium thiomethoxide) is a kind of water white strong alkaline liquid that has an off-odour and can be used for the raw material of pesticides, medicine, Pigment Intermediates, such as pesticide symetryne, Methomyl and organic intermediate raw material; Food additive methionine, vitamin U, vulcanizer raw materia; The antidote of hydrogen sulfide poisoning; Coal gas, natural gas odour additive etc.

Synthesis
Plan A: A production technique for high purity Sodium thiomethoxide, concrete technology step is as follows:
(1) react: the Sodium sulfhydrate adds quantitative mass content of about 32% in the reactor in advance, then the methyl-sulfate of 98% content is dripped to continuous uniform in the reactor, methyl-sulfate and Sodium sulfhydrate reacts and generates thiomethyl alcohol and sodium sulfate, the temperature of reaction controls at about 75 DEG C, and pressure-controlling is at pressure-fired or tiny structure;
(2) alkali liquor absorption: reacted thiomethyl alcohol mixed gas removed through level Four washing and wherein removes wherein moisture content through cyclonic separation again after impurity, pure methyl mercaptan gas after the removal of impurity generates sodium methyl mercaptide solution through two-stage alkali liquor absorption, utilize sodium methyl mercaptide dissolubility difference at different temperatures, rational allocation absorbs alkali content, temperature of reaction of controlling well can generate the sodium methyl mercaptide solution needing concentration, obtains the sodium methyl mercaptide solution of high mass content 40% ~ 50%;
(3) crystallisation by cooling: sodium methyl mercaptide solution crystallization shape solid sodium methyl mercaptide after cooling forms the supersaturated solution under relevant temperature, and this supersaturated solution obtains sodium methyl mercaptide solid after suction filtration;
(4) Dry: After the drying of Sodium thiomethoxide, solid drying equipment is used to obtain the solid Sodium thiomethoxide with a high purity content.
The drying plant used in the steps mentioned above (4) is a pneumatic dryer, infrared drying oven, electric furnace drying baker, or condensate dryer. Domestic sodium methyl mercaptide product mostly adopts NaHS as raw material, react generation methanethiol with dimethyl sulfate or chloromethanes, and then obtain Sodium thiomethoxide product by absorption by liquid caustic soda. Dimethyl sulfate is a severely toxic raw material, and its use, storage, and transport processes have bigger potential safety hazards.
Plan B: Connect a 200-mL Schlenk flask to a vacuum gas manifold. Evacuate the flask and then backfill it with N2 three times, such that the flask is filled with a slightly positive N2 pressure[1].
Take out a block of metallic sodium from the kerosene oil reservoir. Use a paper towel to wipe off the residual oil on the surface, and use a knife to scrape off the oxide layer on the surface. Quickly cut 6.7 g of the metallic sodium (0.29 mol) into small pieces (e.g., about soybean size) and immediately transfer the small pieces to the 200-mL Schlenk flask under a counter flow of N2. Seal the flask with a septum immediately. In addition, to reduce the exposure to air, one can cut out the sodium with some variation from the amount of 6.7 g and adjust the amounts of THF and dimethyl disulfide according to the amount of sodium used.
Transfer 80 mL of an anhydrous, airless THF into the flask via cannula under N2 protection from the Schlenk line. Withdraw 14.0 mL of dimethyl disulfide (0.158 moles; pre-purged with N2) into a syringe and inject it into the flask dropwise under N2 protection.
Replace the septum with a ground glass stopper. Stir the reaction mixture at room temperature for 24 h and then at 60 °C for 3 h. The reaction mixture became viscous while stirring at room temperature; when heated up to 60 °C, the mixture can be stirred more easily, thus speeding up the reaction towards completion.
With the temperature maintained at about 60 °C, use a stream of N2 (0.2 L/min) to blow off most of the THF solvent and the excess dimethyl disulfide until a dry solid appears. Use a cold trap (e.g., acetone/dry ice) to collect the THF and dimethyl disulfide in the outflow.
Evacuate the remaining solid mixture with an oil pump for about 2 h to remove the residual THF and dimethyl disulfide. Then, backfill it with N2 to obtain a light-yellow solid (19.8 g, 97%). Store the solid product Sodium thiomethoxide in a nitrogen atmosphere in the dark.
References:
[1] YONGHE HE. Synthesis of a Thiol Building Block for the Crystallization of a Semiconducting Gyroidal Metal-sulfur Framework.[J]. Jove-Journal of Visualized Experiments, 2018, 134. DOI:10.3791/57455.You may like
Related articles And Qustion
See also
Lastest Price from Sodium thiomethoxide manufacturers
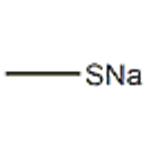
US $1.50/g2025-06-24
- CAS:
- 5188-07-8
- Min. Order:
- 1g
- Purity:
- 99.0% Min
- Supply Ability:
- 10 Tons

US $0.00-0.00/kg2025-04-24
- CAS:
- 5188-07-8
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 20tons