How is Anamorelin Monohydrochloride synthesised?
Synthesis of Anamorelin Monohydrochloride
Anamorelin Monohydrochloride was synthesised starting from 2,2- Dimethylhydrazine by polymerisation of intermediate compounds. The specific synthesis steps are as follows:
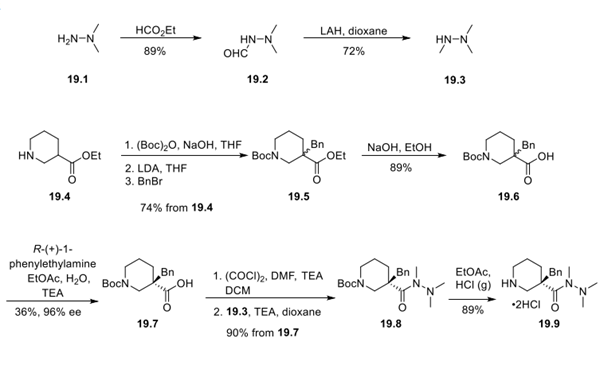
Step 1: Synthesis of Intermediates 19.3 and 19.9
2,2- Dimethylhydrazine (19.1) was treated with ethyl formate to afford N-formyl-dimethylhydrazine 19.2 in good yield. The aldehyde was then reduced with lithium aluminum hydride, and the product 19.3 was isolated as a solution in 1,4- dioxane.
The synthesis of intermediate 19.5 was carried out by first protecting ethyl nipecotate 19.4 as the N-Boc derivative under standard conditions, followed by enolate formation with lithium diisopropylamide at low temperature and dropwise addition of benzyl bromide to provide a racemic mixture of 19.5 in 74% yield over 3 steps. The ester was then saponified to generate acid 19.6. Chiral resolution of the racemic mixture was accomplished by the addition of R-(+)-1-phenethylamine, water, and triethylamine to a solution of 19.6 in EtOAc. Isolation of acid 19.7 as white crystals was done by filtration. The product exhibited 95.8% ee as determined by chiral HPLC.
Significant process research was directed toward the coupling of trimethylhydrazine 19.3 with acid 19.7. This transformation was initially attempted using PyBrop and DIPEA in THF at room temperature but required extended reaction times (48 h) and provided only 65% yield of the expected acyl hydrazide 19.8. Furthermore, isolation of 19.8 was problematic due to the hygroscopic nature of the solid that was obtained under these conditions. CDI and T3P each proved ineffective at amide bond formation and resulted in recovery of the unreacted starting material. EDCI and DMAP in DCM were also attempted but resulted in decomposition products. The synthesis was successfully completed after activating the acid as its acid chloride by treatment with oxalyl chloride, catalytic DMF, and triethylamine, and then reaction with a 5% solution of 19.3 in THF or 1,4-dioxane afforded hydrazide 19.8. The authors noted that the order of addition of reagents was crucial to successful acid chloride formation, and if base was introduced prior to oxalyl chloride or DMF, only 19.7 would be recovered along with decomposition products. Preformation of the acid chloride and subsequent reaction with a solution of 19.3 were found to be critical for kilogram amounts of 19.8 to be obtained in the reported yields (90%). Lastly, the Boc group was removed by dissolving 19.8 in acidic EtOAc to form 19.9 as a white crystalline solid.
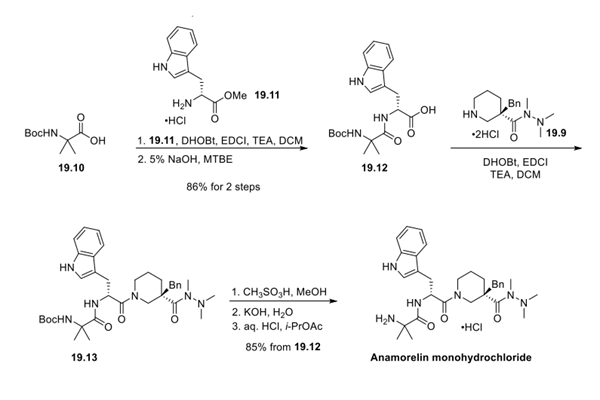
Step 2: Preparation of Anamorelin Monohydrochloride
The synthesis of 19.12 was accomplished by subjecting amino acids 19.10 and 19.11 to standard coupling conditions followed by saponification with 5% NaOH in MTBE to give 19.12 in 86% yield over 2 steps. The same coupling conditions were then employed to couple acid 19.12 and amine 19.9, resulting in amide 19.13. Screening conditions for N-Boc removal revealed that methanesulfonic acid in EtOH or MeOH gave rapid deprotection with minimal byproduct formation. The reaction was quenched with warm aqueous KOH and then allowed to coolslowly, which resulted in crystallization of the free base, which could be isolated in >99.5% purity. Lastly, the hydrochloride salt was formed by dissolving the free base in i-PrOAc and adding a stoichiometric amount of aqueous HCl. The mixture was gently warmed and then cooled to room temperature to afford crystalline anamorelin monohydrochloride.