How are SF6 Lewis structures and hybrids formed
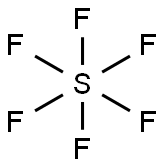
SF6 Lewis structure
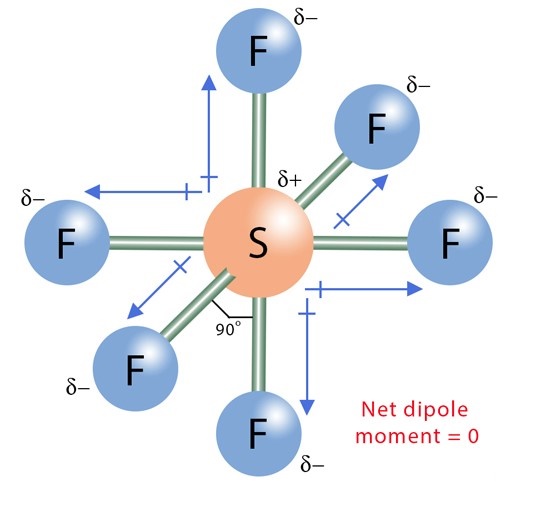
The SF6 Lewis structure is made up of one sulfur atom (S) and six fluorine atoms (F). Sulfur atom (S) is the central atom, fluorine atom (F) is the external atom, sulfur atom (S) and each fluorine atom (F) are connected by a single bond, each fluorine atom (F) has three lone pairs of electrons, and the central atom is symmetrically distributed around. The SF6 bond angle is 90 degrees. The SF6 Lewis structure is shown below:
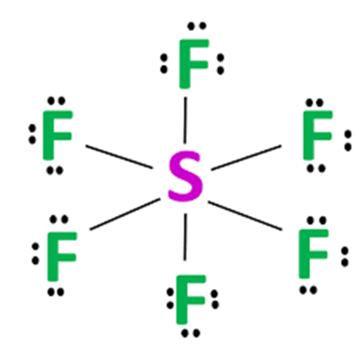
Steps for drawing the SF6 Lewis structure
Step 1 Calculate the number of valence electrons for S and F
Based on the information in the periodic table, we are able to obtain: Sulphur (S) and fluorine (F) are in the 16th and 17th group of the periodic table. Therefore, the valence electrons of the sulfur (S) and fluorine (F) atoms are 6 and 7, respectively, so the total number of valence electrons in the SF6 molecule = 1 valence electron from the sulfur atom + 6 valence electrons from the fluorine atom = 6 + 7(6) = 48.
Step 2 Identify the central atom
The central atom must have a high valence or minimal electronegativity. For the SF6 molecule, sulfur has a maximum valence of 6 and fluorine has a maximum valence of 1; and Sulfur has a lower electronegativity than oxygen, so the sulfur atom is the central atom and the fluorine atom is the outer atom.
Step 3 Labelling the electron lone pairs between atoms
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells, total electron pairs are determined by dividing the number total valence electrons by two. For SF6 molecule, Total number of pairs of electrons are 24.
The sulphur atom is connected to each of the six fluorine atoms by a σ-bond (one σ-bond equals one electron pair), and the remaining 18 electron pairs are distributed as follows: three electron pairs on each fluorine atom. These lone electrons repel each other and maintain symmetry around the central atom.
Step 4 Stabilising the Lewis structure
When many atoms in an ion or molecule are positively or negatively charged, or when there are more charges on the atoms (e.g. +2, +3, -2, -3), the structure is unstable. Therefore, if possible, we should try to minimise the charges on the atoms. But in the SF6 molecule, we don't need to reduce the charge on the atoms because there are more than 8 electrons in the outer shell of sulphur, the reason is that some atoms can expand their valence shells to accommodate more electrons to achieve a stable structure. And the external fluorine atoms have formed an octet, so they are also stable.
SF6 hybridisation
In the SF6 molecule, the total number of electrons in the sulphur is 16, so the shell layer is populated with different energy levels depending on its capacity and level of hierarchy. The hybridisation of sulphur is then 3s2 3p4. The hybridisation of fluorine is 2s2 2p5.
When these two atoms are bonded together and the compound enters the excited state, the electrons of the sulphur move from energy level p to energy level d. These orbitals overlap with the 2p orbitals of the fluorine atom. This creates six hybrid orbitals (one 3s, three 3p and two 3d). These hybrid orbitals can accommodate shared electrons. These six orbitals are located in the six directions of the octahedral shape. Therefore, the hybridisation of SF6 is sp3d2.