Hexanophenone: Overview, Appliations in Parmaceutical Cemistry and Safety
General Description
Hexanophenone, an organic compound with a carbonyl group attached to a phenyl ring and a hexyl chain, is widely used in the fragrance and chemical industries. Its synthesis involves the condensation of hexyl halide with phenylmagnesium bromide, yielding a compound with a distinct odor and solubility in organic solvents. In pharmaceutical chemistry, hexanophenone derivatives have shown promise in developing potent fungicidal agents through structural modifications. However, due to its flammability and health risks, strict safety measures, including proper ventilation, protective gear, and storage precautions, are essential during handling. Overall, while hexanophenone demonstrates significant potential in diverse applications, its safe usage is paramount to prevent adverse effects on health and the environment.

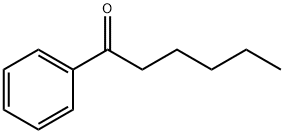
Figure 1. Hexanophenone
Overview
Hexanophenone, also known as hexyl phenyl ketone, is an organic compound belonging to the ketone family. It is characterized by the presence of a carbonyl group attached to a phenyl ring and a hexyl chain. This molecular structure confers unique properties to hexanophenone, making it a valuable chemical intermediate in various industrial applications. The compound exhibits a distinct odor and is soluble in organic solvents. Its synthesis typically involves the condensation of hexyl halide with phenylmagnesium bromide, followed by acidification to yield the desired ketone. Hexanophenone finds use in the fragrance industry, where it contributes to the scent profiles of perfumes and cosmetics. Additionally, it serves as a building block in the synthesis of complex organic molecules and plays a role in the production of certain polymers. However, it's worth noting that hexanophenone should be handled with caution due to its potential hazards, including flammability and skin irritation. Therefore, appropriate safety measures must be taken when working with this compound. In summary, hexanophenone is a versatile ketone with diverse applications in the fragrance and chemical industries, but its handling requires careful attention to safety. 1
Applications in Pharmaceutical Chemistry
Hexanophenone is a versatile compound with various applications in pharmaceutical chemistry. In a study, 4-(azol-1-ylmethyl)-2-alkyl-2-aryl-1,3-dioxolanes, which were synthesized and studied for their fungicidal activity, likely involve hexanophenone derivatives as key components. In pharmaceutical chemistry, hexanophenone and its derivatives have been explored for their potential fungicidal properties. The synthesis of 4-(azol-1-ylmethyl)-2-alkyl-2-aryl-1,3-dioxolanes involved the modification of hexanophenone by attaching azole groups and other alkyl/aryl substituents. These modifications can alter the compound's lipophilicity and bioactivity, contributing to enhanced fungicidal properties. The study indicates that some of these synthesized compounds exhibited higher fungicidal activity than the standard triadimefon. This suggests that the incorporation of hexanophenone derivatives into the dioxolane structure led to potent fungicidal effects. Furthermore, the study revealed a correlation between the lipophilicity of the compounds and their inhibition of pathogenic fungi mycelium growth, highlighting the significance of molecular structure in determining fungicidal activity. Overall, the application of hexanophenone derivatives in the synthesis of 4-(azol-1-ylmethyl)-2-alkyl-2-aryl-1,3-dioxolanes demonstrates the potential of this compound in pharmaceutical chemistry, particularly in the development of novel fungicidal agents with improved efficacy against phytopathogenic fungi. 2
Safety
Hexanophenone is a hazardous laboratory chemical due to its flammability and potential health risks. Safety precautions must be strictly followed when handling this substance. Prior to use, it is essential to obtain special instructions and wear appropriate personal protective equipment. Exposure to Hexanophenone can lead to drowsiness or dizziness, emphasizing the need to avoid inhalation of dust, fumes, or vapors. Skin contact should be followed by thorough washing, and ingestion or smoking while using Hexanophenone should be strictly prohibited. Utilize the chemical outdoors or in well-ventilated areas, keeping containers tightly sealed and away from sources of heat or ignition. Grounding and bonding equipment is crucial to prevent static discharge. In case of exposure, immediate medical attention is necessary. Adhering to proper handling, storage, and disposal protocols is vital for ensuring safety and minimizing health and environmental risks associated with Hexanophenone. 3
Reference
1. Hexanophenone. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 70337.
2. Talismanov VS, Popkov SV. Synthesis and fungicidal activity of 4-(azol-1-ylmethyl)-2-alkyl-2-aryl-1,3-dioxolanes. Agrokhimiya. 2007; 5: 53-57.
3. SAFETY DATA SHEET: 2-Hexanone. 2021; CAS No: 591-78-6.
You may like
Related articles And Qustion
See also
Lastest Price from Hexanophenone manufacturers

US $1.90/KG2025-06-10
- CAS:
- 942-92-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50 ton

US $10.00/ASSAYS2025-05-04
- CAS:
- 942-92-7
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 ton