Hexamethyldisilazane: Application and synthesis
General description

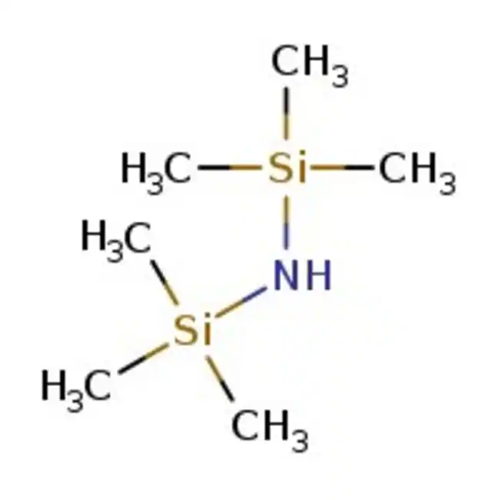
Figure 1 Appearance of hexamethyldisilazane.
Application
Hexamethyldisilazane is used as a reagent in condensation reactions of heterocyclic compounds such as in the microwave synthesis of a derivative of xanthine.[1] The hexamethyldisilazane mediated trimethylsilylation of alcohols, thiols, amines and amino acids as protective groups or for intermediary organosilicon compounds is found to be very efficient and replaced TMSCl reagent. Silylation of glutamic acid with excess hexamethyldisilazane and catalytic TMSCl in either refluxing xylene or acetonitrile followed by dilution with alcohol (methanol or ethanol) yields the derived lactam pyroglutamic acid in good yield. Hexamethyldisilazane in the presence of catalytic iodine facilitates the silylation of alcohols in excellent yields. It can be used to silylate laboratory glassware and make it hydrophobic, or automobile glass, just as Rain-X does. In gas chromatography, hexamethyldisilazane can be used to silylate OH groups of organic compounds to increase volatility, this way enabling GC-analysis of chemicals that are otherwise non-volatile.
In addition, in photolithography, hexamethyldisilazane is often used as an adhesion promoter for photoresists. Best results are obtained by applying hexamethyldisilazane from the gas phase on heated substrates.[2] In electron microscopy, hexamethyldisilazane can be used as an alternative to critical point drying during sample preparation.[3] In plasma-enhanced chemical vapor deposition (PECVD), it is used as a molecular precursor as a replacement to highly flammable and corrosive gasses like SiH4, CH4, NH3 as it can be easily handled. Hexamethyldisilazane is used in conjunction with plasma (physics) of various gases such as argon, helium and nitrogen to deposit SiCN thin films/coatings with excellent mechanical, optical and electronic properties. In pyrolysis-gas chromatography-mass spectrometry, it is added to the analyte to create silylated diagnostic products during pyrolysis, in order to enhance detectability of compounds with polar functional groups.[4]
Synthesis
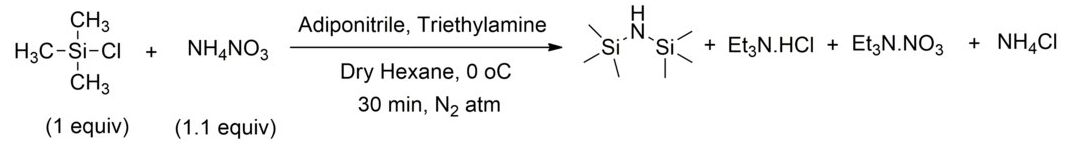
As previous work described, the hexamethyldisilazane can be synthesized by the following steps.[5] In a pressure reactor (70 L volume) of corrosion-resistant material (Hastelloy B3, Hastelloy steels are generally suitable for the B-and C-series) streams of ammonia (31.3 kg/h) and trimethylchlorosilane (8.0 kg/h) each preheated at 175°C, are reacted together at a pressure of 230 bar. The mean residence time in the reaction part is 35 minutes. In a first separation stage downstream, at a pressure of 160 bar and a temperature of 160 °C from the heavy two-phase reaction mixture, lower phase A is separated and extracted with 3.0 kg/h of pure ammonia. The extracted phase A is then transferred to a drying apparatus under catharsis, and the phase in 3.9 kg/h solid salt and gaseous ammonia, which is recycled, is decomposed. The purity of the ammonium chloride is 99.9 wt-%.
The organosilazane ammonia-containing stream B exiting the first separation stage is decompressed at 50 bar before it enters the second separation step and heated to 100 °C, which again forms a two-phase mixture. The heavy, lower organosilazane and ammonia containing C phase is separated from the ammonia-D flow with decompression, where the ammonia escapes as a gas and recycled. The ammonium-containing organosilazane is freed in a degassing tank of dissolved ammonia in vacuum. The resulting product flow of 5.7 kg/h (96% of theory) consists of hexamethyldisilazane with a purity of 99.0 wt-%.
References
[1]Burbiel et al. (2006). Microwave-assisted ring closure reactions: Synthesis of 8-substituted xanthine derivatives and related pyrimido- and diazepinopurinediones. Beilstein J Org Chem. 2: 20.
[2]Cornell NanoScale Science & Technology Facility. CNF - Photolithography Resist Processes and Capabilities. Retrieved 2008-01-29.
[3]Bray et al. (1993). Comparison of hexamethyldisilazane (HMDS), Peldri II, and critical-point drying methods for scanning electron microscopy of biological specimens. Microsc. Res. Tech. 26 (6): 489–95.
[4]Giuseppe et al. (2001). Gas chromatographic–mass spectrometric analysis of products arising from pyrolysis of amino acids in the presence of hexamethyldisilazane. Journal of Chromatography A. 922 (1–2): 235–241.
[5]Bauer. (2006). Improved high-pressure continuous process for preparation of organosilazanes by reaction of organohalosilanes with ammonia with subsequent liquid-liquid phase separation of the reaction products. Patent Number: DE102004051899.
You may like
Related articles And Qustion
See also
Lastest Price from Hexamethyldisilazane manufacturers

US $1.70/kg2025-06-09
- CAS:
- 999-97-3
- Min. Order:
- 10kg
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $10.00/KG2025-04-21
- CAS:
- 999-97-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt