Hexamethyldisilazane -a Widely Used Organosilicon Compound
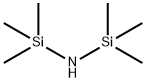
Hexamethyldisilazane, also known as HMDS and bis(trimethylsilyl)amine, is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. An electron diffraction study shows that silicon-nitrogen bond length (173.5 pm) and Si-N-Si bond angle (125.5o) to be similar to disilazane (in which methyl groups are replaced by hydrogen atoms) suggesting that steric factors are not decision in regulating angles [1]. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry. Additionally, HMDS is also increasingly used as molecular precursor in chemical vapor deposition techniques to depositing silicon carbonitride thin films or coatings [2].
Hexamethyldisilazane is an N-silyl compound obtained from ammonia by replacement of two of the hydrogens with trimethylsilyl groups. Hexamethyldisilazane is a derivatisation agent used in gas chromatography mass spectrometry applications. It has a role as a chromatographic reagent. It derives from a hydride of an ammonia. Hexamethyl disilazane appears as a liquid. May be toxic by ingestion. Irritates skin and eyes. Vapors are heavier than air. May emit highly toxic nitrogen oxide fumes when heated to decomposition. Used to make other chemicals.
Hexamethyldisilazane is synthesized by treatment of trimethylsilyl chloride with ammonia:
2 (CH3)3SiCl + 3 NH3 → [(CH3)3Si]2NH + 2 NH4Cl
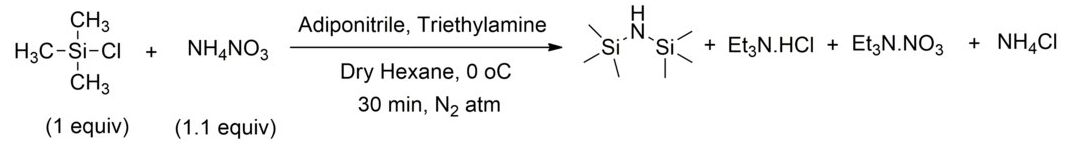
Ammonium nitrate together with triethylamine can be used instead [3]. This method is also useful for 15N isotopic enrichment of HMDS.
Alkali metal bis(trimethylsilyl)amides result from the deprotonation of bis(trimethylsilyl)amine. For example, lithium bis(trimethylsilyl)amide (LiHMDS) is prepared using n-butyllithium:
[(CH3)3Si]2NH + BuLi → [(CH3)3Si]2NLi + BuH
LiHMDS and other similar derivatives: sodium bis(trimethylsilyl)amide (NaHMDS) and potassium bis(trimethylsilyl)amide (KHMDS) are used as a non-nucleophilic bases in synthetic organic chemistry.
Among amines, hexamethyldisilazane (HMDS), in particular, is found to cause contamination problems. HMDS is one of the primary sources of ammonia, and it is by itself an amine and an organic compound.
HMDS is used in the lithography process to treat the surface of a Si wafer (silane coupling agent). HMDS, reacting (silane coupling reaction) with the surface of metals and ceramics, forms hydrophilic trimethylsilanol (TMSiOH) on the surface. This makes it easier to coat a Si wafer surface with photoresist.
2{-Si-OH} + (CH3)3-Si-NH-Si- (CH3)3 =2{Si-OH-Si(CH3)3} + NH3
HMDS also reacts with moisture in the air and gradually converts to NH3 and methyl silanol.
(CH3)3-Si-NH-Si- (CH3)3 + H2O = 2(CH3)3-Si-OH + NH3
Therefore, HMDS must be considered as one of the primary sources of NH3. When excessive HMDS exists in the cleanroom air, it will induce unwanted reactions with the surface of a Si wafer or a glass substrate to form a trimethylsiloxy bond (this is not adhesion but surface reaction), which results in persistent organic contamination. NH3 and TMSiOH show a strong correlation in some cases, while they do not show any correlation at all in other cases. Figure 1 shows the relationship between NH3 and TMSiOH.
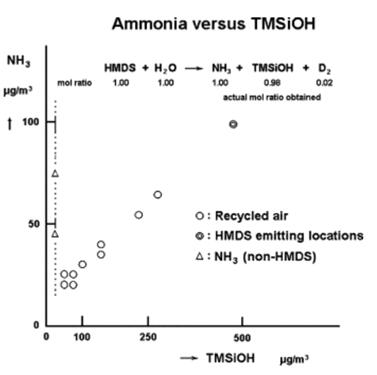
Figure 1. Scattering map of the relationship between NH3 gas and TMSiOH based on analysis of cleanroom air contaminated by these compounds.
HMDS generates NH3 which induces particle generation and the T-top phenomenon on chemically amplified photoresist. Also, HMDS forms the (CH3)3–Si–O-group on Si wafer surface and on glass surface which changes these surfaces to lipophilic and indirectly facilitates formation of a surface susceptible to adhesion of organic impurities. The organic compounds adhering to the surface tend to decompose, causing particle generation or problems in the film deposition process. Specifically, they may cause abnormal colors, haze and clouding on glass which adversely affects the optical properties and the display function. When a glass surface is irradiated with light such as UV radiation, the (CH3)3–Si–O-group is decomposed due to photoreaction and precipitates SiO2. The SiO2 precipitates may make the glass cloudy, generate particles, or cause other problems on the glass surface [4].
Hexamethyldisilazane is employed as a reagent in many organic reactions. HMDS is used as a reagent in condensation reactions of heterocyclic compounds such as in the microwave synthesis of a derivative of xanthine. The HMDS mediated trimethylsilylation of alcohols, thiols, amines and amino acids as protective groups or for intermediary organosilicon compounds is found to be very efficient and replaced TMSCl reagent. Silylation of glutamic acid with excess hexamethyldisilazane and catalytic TMSCl in either refluxing xylene or acetonitrile followed by dilution with alcohol (methanol or ethanol) yields the derived lactam in good yield. HMDS in the presence of catalytic iodine facilitates the silylation of alcohols in excellent yields. HMDS can be used to silylate laboratory glassware and make it hydrophobic, or automobile glass, just as Rain-X does. In gas chromatography, HMDS can be used to silylate OH groups of organic compounds to increase volatility, this way enabling GC-analysis of chemicals that are otherwise non-volatile [2].
In photolithography, HMDS is often used as an adhesion promoter for photoresists. Best results are obtained by applying HMDS from the gas phase on heated substrates.
In electron microscopy, HMDS can be used as an alternative to critical point drying during sample preparation.
In pyrolysis-gas chromatography-mass spectrometry, HMDS is added to the analyte to create silylated diagnostic products during pyrolysis, in order to enhance detectability of compounds with polar functional groups [5].
In plasma-enhanced chemical vapor deposition (PECVD), HMDS is used as a molecular precursor as a replacement to highly flammable and corrosive gasses like SiH4, CH4, NH3 as it can be easily handled. HMDS is used in conjunction with plasma (physics) of various gases such as argon, helium and nitrogen to deposit SiCN thin films/coatings with excellent mechanical, optical and electronic properties.
References
[1] D.A. Armitage (1982). "Organosilicon - an overview". Comprehensive Organometallic Chemistry: 1–203. doi:10.1016/B978-008046518-0.00014-3
[2] https://en.wikipedia.org/wiki/Bis(trimethylsilyl)amine
[3] S.V. Chernyak; Yu. G. Yatluk; A.L. Suvorov (2000). "A Simple Synthesis of Hexamethyldisilazane (Translated from Zhurnal obshcheĭ khimiĭ, Vol. 70. No. 8, 2000. p1401)". Russian Journal of General Chemistry. 70: 1313.
[4] Developments in Surface Contamination and Cleaning (Second Edition) Volume 1: Fundamentals and Applied Aspects, 2008, Pages 197-329. Chapter 7 - Airborne Molecular Contamination: Contamination on Substrates and the Environment in Semiconductors and Other Industries
[5] Giuseppe Chiavari; Daniele Fabbri & Silvia Prati (2001). "Gas chromatographic–mass spectrometric analysis of products arising from pyrolysis of amino acids in the presence of hexamethyldisilazane". Journal of Chromatography A. 922 (1–2): 235–241. doi:10.1016/S0021-9673(01)00936-0. PMID 11486868.
You may like
Related articles And Qustion
Lastest Price from Hexamethyldisilazane manufacturers

US $1.70/kg2025-06-09
- CAS:
- 999-97-3
- Min. Order:
- 10kg
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $10.00/KG2025-04-21
- CAS:
- 999-97-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt