HATU:a third-generation coupling reagent
Description
HATU, the N-guanidium salt of HOAt, has been recently utilized in the macrocyclization of complicated molecules.
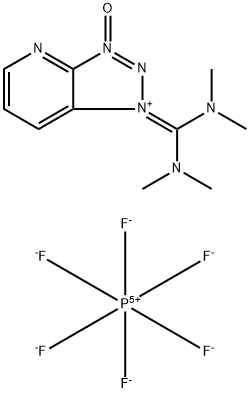
Hexafluorophosphate aza benzotriazole tetramethyl uronium (HATU), IUPAC name (N-{(dimethylamino)-1H-1,2,3-triazo-lo[4,5-b]pyridin-1-ylmethylene}-N-methyl methanaminium hexafluorophosphate N-oxide) is used in both small molecule’s synthesis or peptide synthesis. Louis A. Carpino discovered a new ester derivative of 1-hydroxy-7-azabenzotriazole (HOAt) in 1993, which occurs in uranium salt and iminium salt[1].
Uses
HATU was a third-generation coupling reagent with the ability to decrease racemization. HATU causes amine acylation or the formation of an amide bond. In addition to nucleophiles, it is employed in peptide cyclization. HATU catalyzes the nucleophilic addition process. In terms of the coupling reaction refers to the coupling of the same fragment, whereas cross-coupling refers to the coupling of two separate fragments. The mechanism of HATU involves the activation of the carboxylic group through the formation of a carboxylate anion that attacks HATU to produce O-acyl(tetramethyl)isouronium salt, which then proceeds the addition of the nucleophile, i.e., amines.
HOAt and its corresponding uronium (HATU) and phosphonium salts were shown to be superior to their benzotriazole analogues in solid-phase peptide synthesis involving hindered amino acids when coupling times were shortened, and excesses of reagents were reduced in order to emphasize the differences. HATU was the optimal reagent for the acylation of support-bound secondary amines in the solid-phase synthesis of β-turn mimetics. HATU and HOAt have been used for inverse amide bond formation between a support-bound carboxylic acid and an amino ester in solution[2].
Since Nielsen first introduced PNA (peptide nucleic acid), in which the sugar-phosphate backbone was replaced by a polyamide chain composed of aminoethylglycine covalently linked to DNA bases, several peptide coupling reagents have been employed in the synthesis of PNAs as DNA mimics. For example, the coupling reaction between TL-Phe and L-Val-OMe using TDBTU, DEPBT, HBTU, or HATU produced the chiral PNA monomer with good enantiomeric purity.
Ehrlich investigated the cyclization of all-L-tetrapeptides or all-L-pentapeptides. It needed caution during the reaction to avoid side reactions such as cyclodimerization or epimerization at the C-terminal residue. HOAt-based reagents such as HATU, HAPyU, and TAPipU were more effective than TBTU or BOP. Notably, a-configuration alternation of the linear precursor ensured efficient cyclization. Marchelli reported a successful optimization of the coupling condition using HATU/collidine in synthesising PNA analogues both in solution and in the solid phase. Giralt and Lloyd-Williams applied HATU and improved the synthetic procedure of dehydrodidemnin B based on an earlier total synthesis of didemnins.
Toxicity
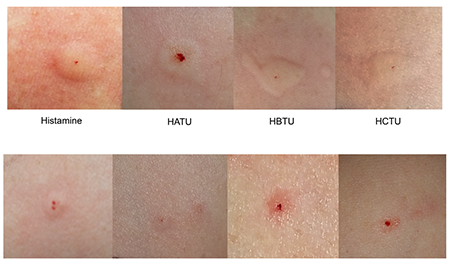
HOAt was nontoxic to rats when dosed at 2000 mg kg−1 via the oral route. HOAt was not a skin irritant in rabbits. In rabbits, HOAt was found to be a slight eye irritant. Studies in guinea pigs revealed that HOAt did not induce contact allergy. HOAt did not induce micronuclei in bone marrow polychromatic erythrocytes in male mice when evaluated in the in vivo micronucleus assay[3].
HATU showed no mortality but limited toxicity to rats when dosed at 2000 mg kg−1 via the oral route. HATU was not a skin irritant in rabbits. In rabbits, HATU was found to be a slight eye irritant. Studies in guinea pigs revealed that HATU-induced contact allergy.
PyAOP was nontoxic to rats when dosed at 2000 mg kg−1 via the oral route. PyAOP was not a skin irritant in rabbits. In rabbits, PyAOP was found to be a very severe eye irritant. Studies in guinea pigs revealed that PyAOP induced contact allergy.
References:
[1] CHAWLA P A, SHOME A, JHA K T. Hexafluorophosphate Azabenzotriazole Tetramethyl Uronium (HATU): A Unique Cross-Coupling Reagent[J]. SynOpen, 2023, 66 2. DOI:10.1055/s-0042-1751499.[2] SO-YEOP HAN Y A K. Recent Development of Peptide Coupling Reagents in Organic Synthesis[J]. ChemInform, 2004, 35 26. DOI:10.1002/chin.200426219.
[3] KIM S. Peptide Coupling Agents[C]. 1900. DOI:10.1016/B978-0-12-386454-3.00642-4.
You may like
Related articles And Qustion
See also
Lastest Price from HATU manufacturers

US $0.00/g2026-03-03
- CAS:
- 148893-10-1
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 9999

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 148893-10-1
- Min. Order:
- 1KG
- Purity:
- 99.5%min
- Supply Ability:
- 1000KGS