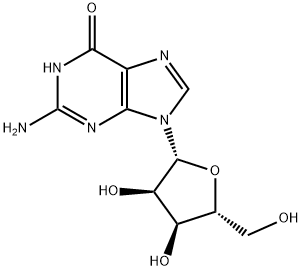
Guanosine Hydrate: A Versatile Nucleotide Derivative Enhancing Photochemical Reactions and Cellular Functions
General Description
Guanosine hydrate, a nucleotide derivative, significantly impacts photochemical reactions involving quinones like 9,10-anthraquinone and 2-methyl-1,4-naphthoquinone. It actively participates in photoinduced electron transfer and hydrogen abstraction, showcasing its versatility compared to guanine.

Guanosine is a purine nucleoside thought to have neuroprotective properties. It is released in the brain under physiological conditions and even more during pathological events, reducing neuroinflammation, oxidative stress, and excitotoxicity, as well as exerting trophic effects in neuronal and glial cells. In agreement, guanosine was shown to be protective in several in vitro and/or in vivo experimental models of central nervous system (CNS) diseases, including ischemic stroke, Alzheimer’s disease, Parkinson’s disease, spinal cord injury, nociception, and depression. The mechanisms underlying the neurobiological properties of guanosine seem to involve the activation of several intracellular signalling pathways and close interaction with the adenosinergic system, with consequent stimulation of neuroprotective and regenerative processes in the CNS.
Uses
Compared with the pyrimidine bases of nucleic acids, the purines are relatively inert to photochemical alteration. No photoproducts derived specifically from adenine or guanine have been isolated from ultraviolet-irradiated DNA or RNA. However, purine bases and nucleosides can be substituted at C8 by free radical species generated photochemically from simple alcohols, amines, and ethers, and these reactions may be relevant to the radiation-induced cross-linking of proteins and other biological molecules to nucleic acids. Researchers report that guanosine is substituted in a similar manner by the free radicals produced on photolysis of 8-bromopurine nucleosides to give compounds in which two purine nucleoside moieties are coupled together through their respective C8 positions.
Guanosine, a natural nucleoside, is an important low-molecular-weight building block for supramolecular hydrogels due to its unique self-assembly properties. This nucleoside containing the natural nucleobase purine provides multiple edges for hydrogen-bonding interactions. The self-complementary hydrogen-bonding donors (N1 amide and N2 amino) and acceptors (N7, N3 and O6) enable guanosine and its derivatives to self-assemble into dimers, ribbons, sheets, or macrocycles via noncanonical base pairing. Most guanosine-based hydrogels are based on the supramolecular assembly of macrocyclic G-quartet units. This macrocyclic structure generates a central cavity where four carbonyl oxygens (O6) provide potential sites for cation coordination (typically Na+, K+), thus providing cation-induced stability to the columnar aggregates of G-quartets that immobilize significant amount of water to form a hydrogel.
The biocompatible and biodegradable properties of guanosine and its derivatives provide a diverse toolbox in biomedicinal research, particularly in intracellular delivery of drug molecules. More importantly, the ease of synthetic derivatization of guanosine enables tuning the functionality and variability of guanosine-derived supramolecular hydrogels.
References:
[1] LUIS E B BETTIO A L S R Joana Gil Mohapel. Guanosine and its role in neuropathologies.[J]. GEOJOURNAL, 2016, 20 1: 54. DOI:10.1007/s11302-016-9509-4.[2] S. N. BOSE. Photochemical (8→8) coupling of purine nucleosides to guanosine[J]. Nature, 1978, 271 5647. DOI:10.1038/271783a0.
You may like
See also
Lastest Price from Guanosine manufacturers

US $0.00/kg2025-08-18
- CAS:
- 118-00-3
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 2000kgs

US $10.00/Kg2025-04-21
- CAS:
- 118-00-3
- Min. Order:
- 1Kg
- Purity:
- 98%
- Supply Ability:
- 20Ton