Graphene Oxide: Introduction and Market News
What is graphene?
Graphene is a material made of carbon atoms that are bonded together in a repeating pattern of hexagons. Graphene is so thin that it is considered two dimensional. Graphene's flat honeycomb pattern gives it many extraordinary characteristics, such as being the strongest material in the world, as well as one of the lightest, most conductive and transparent. Graphene has endless potential applications, in almost every industry (like electronics, medicine, aviation and much more).
The single layers of carbon atoms provide the basis for many other materials. Graphite, like the substance found in pencil lead, is formed by stacked graphene. Carbon nanotubes are made of rolled graphene and are used in many emerging applications from sports gear to biomedicine.
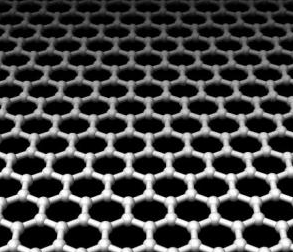
What is graphene oxide?
As graphene is expensive and relatively hard to produce, great efforts are made to find effective yet inexpensive ways to make and use graphene derivatives or related materials. Graphene oxide (GO) is one of those materials - it is a single-atomic layered material, made by the powerful oxidation of graphite, which is cheap and abundant. Graphene oxide is an oxidized form of graphene, laced with oxygen-containing groups. It is considered easy to process since it is dispersible in water (and other solvents), and it can even be used to make graphene. Graphene oxide is not a good conductor, but processes exist to augment its properties. It is commonly sold in powder form, dispersed, or as a coating on substrates.
Graphene oxide is synthesized using four basic methods: Staudenmaier, Hofmann, Brodie and Hummers. Many variations of these methods exist, with improvements constantly being explored to achieve better results and cheaper processes. The effectiveness of an oxidation process is often evaluated by the carbon/oxygen ratios of the graphene oxide.
Graphene oxide uses
Graphene Oxide films can be deposited on essentially any substrate, and later converted into a conductor. This is why GO is especially fit for use in the production of transparent conductive films, like the ones used for flexible electronics, solar cells, chemical sensors and more. GO is even studied as a tin-oxide (ITO) replacement in batteries and touch screens.
Graphene Oxide has a high surface area, and so it can be fit for use as electrode material for batteries, capacitors and solar cells. Graphene Oxide is cheaper and easier to manufacture than graphene, and so may enter mass production and use sooner.
GO can easily be mixed with different polymers and other materials, and enhance properties of composite materials like tensile strength, elasticity, conductivity and more. In solid form, Graphene Oxide flakes attach one to another to form thin and stable flat structures that can be folded, wrinkled, and stretched. Such Graphene Oxide structures can be used for applications like hydrogen storage, ion conductors and nanofiltration membranes.
Graphene oxide is fluorescent, which makes it especially appropriate for various medical applications. bio-sensing and disease detection, drug-carriers and antibacterial materials are just some of the possibilities GO holds for the biomedical field.


