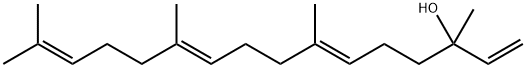
Geranyl linalool—an essential aroma ingredient

Irritant to eyes, respiratory tract and skin, geranyl linalool is a colorless and transparent liquid. Structurally, geranyl linalool is a straight chain diterpenoid. Being bioactive natural product, geranyl linalool is the essential ingredients that determine the spice type. In the other hand, geranyl linalool was also an important intermediate for Teprenone, a gastric mucosa protective agent. Employing geranyl linalool as substrate, the most convenient synthesis method of Teprenone would be achieved. Catalyzed by isopropanol aluminum and reacted with methyl acetoacetate, Teprenone could be obtained in one step. Geranyl linalool can also be used as insect pheromone and natural insect insecticide. So, geranyl linalool possesses important economic value.
Preparation of geranyl linalool: taking (E) - nerolidol as raw material, protection of the hydroxyl was conducted with dihydropyran to give the tetrahydropyran (E) – nerolidol ether. Then, with the presence of selenium dioxide and tert-butyl hydroperoxide (TBHP), selective oxidation of the methyl located at the trans-position of the ether was taken place. Oxidation product, hydroxylation of trans allyl, (E, E) – 1, 2 - hydroxy nerolidol tetrahydropyran ether was obtained. Through the halogenation reaction, (E, E)-1, 2- halogenated nerolidol tetrahydropyran ether was obtained. Reacted with isopropyl methyl ketone, achieved by deprotonation of lithium diisopropylamide, (6E, 10E)-2, 6, 10, 14 - tetramethyl - 14 - (tetrahydropyran -2 - oxygen) -6, 10, 15-3- triene -3 – hexadecanone would be obtained. Reduced by sodium borohydride, the corresponding alcohol could be obtained. Sulfonylation of the alcohol would take place to give the sulphonate under the catalyzed by base in the presence of sulfonic acid chloride. Then, elimination of the sulfonate groups would give the (E, E) -geranyl linalool tetrahydropyran ether, which could product (E, E) -geranyl linalool with further deprotection. Since the configuration of the tertiary carbon at 3-position of (E) - nerolidol is not affected during the reaction, the optically active (E, E) - geranyl linalool could be obtained by using the optically active (E) - nerolidol.
References
[1]. D. W. Dockery et al., An association between air pollution and mortality in six U.S. N. Engl. J. Med. 329, 1753–1759 (1993)
You may like
Related articles And Qustion
See also
Lastest Price from Geranyl linalool manufacturers

US $1.00/kg2025-04-21
- CAS:
- 1113-21-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $75.00-20.00/kg2025-04-15
- CAS:
- 1113-21-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton