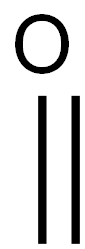General Facts related to Paraformaldehyde
Paraformaldehyde is a smallest polymerization product of formaldehyde. It possesses the similar features of formaldehyde, which has with a wider range of applications. Paraformaldehyde is extensively used in the manufacturing of synthetic resins like phenolic resins, thiourea, melamine formaldehyde resins, and as a substitute of formalin to generate the resinous binding material. Paraformaldehyde is usually used together with similar chemical compounds like urea, melamine, phenol, resorcinol, tannin or other reactants in the manufacturing of particle board, fibreboard and plywood.

Production of Paraformaldehyde
Paraformaldehyde is a produced as a white precipitate by condensation from the main species of methanediol in solution of formaldehyde which is also known as formalin. The solution is chiefly of oligomers, but when it becomes large, the material turn into sufficiently insoluble as to precipitate. The reaction is determined to the left, in order to release formaldehyde, by a low concentration of formaldehyde, and quickened by acidic or alkaline conditions. Solid paraformaldehyde has odour of formaldehyde monomer, so it is fundamentally a convenient means of delivering formaldehyde slowly.
Toxic Effects of Paraformaldehyde
Paraformaldehyde is quite toxic by skin contact. It has recently been named as a potential probable human carcinogen.
Paraformaldehyde may cause skin itching and rash that leads to skin allergy when it is extensively exposed. It has moreover been reported to cause reproductive and mutagenic problems in humans when exposed more.
Applications in Chemical Industry
A vast solid form of formaldehyde is a extremely useful reagent in classical organic reactions. Several applications have been discovered in the chemical industry and organic chemistry research laboratories. Paraformaldehyde is used in organic reactions with transition metals as the catalysts. These methodologies, paraformaldehyde has been used as methylene blocks, hydroxymethylation reagents, CO source, syngas surrogate, hydrogen donor or acceptor, formylation and methylation reagents.
Moreover, in recent researches has reported organocatalyzed transformations of paraformaldehyde have been included as well.In some instance paraformaldehyde is depolymerized, the resultant formaldehyde may be make use as a disinfectant, fungicide, and fixative. Longer chain-length polyoxymethylenes are used as a thermoplastic and are known as polyoxymethylene plastic.
Paraformaldehyde finds diversified usage in chemical industry which can be as both intermediate and a range of fields such as cleaning products, fluorescent lights, organic chemical synthesis, personal care products, and also in consumer goods.
You may like
See also
Lastest Price from Paraformaldehyde manufacturers

US $0.00/kg2025-05-27
- CAS:
- 30525-89-4
- Min. Order:
- 230kg
- Purity:
- 99%
- Supply Ability:
- 200000

US $10.00/KG2025-04-21
- CAS:
- 30525-89-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt