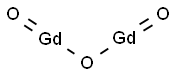Gadolinium Oxide: Optical Properties, Synthesis Routes and Toxicity
General Description
Gadolinium oxide is a highly promising material with exceptional optical properties, ideal for imaging applications. Its stable trivalent state in the matrix creates strong paramagnetic characteristics, beneficial for contrast agents in imaging. The compound's intrinsic optical features enable sharp wavelength absorptions, photostability, and doping with lanthanide ions further enhances its optical properties. Various synthesis routes, such as sonic-chemical, solid-state technic, and sol-gel methods, produce nanoparticles suitable for different applications. However, Gadolinium oxide is toxic, causing eye irritation, harm to aquatic life, and fibrogenic effects. Proper handling is crucial to prevent environmental harm and health risks.

Figure 1. Gadolinium Oxide
Optical Properties
Gadolinium oxide is a highly promising material known for its exceptional optical properties, particularly in the realm of imaging applications. As a rare-earth oxide, Gadolinium oxide serves as an ideal host lattice due to its chemical and thermal stability, low phonon energy, and ability to be doped with lanthanide ions. The trivalent state of gadolinium within the matrix creates a stable 4f shell with seven unpaired spins, resulting in strong paramagnetic characteristics that are advantageous for contrast agents in magnetic resonance and fluorescence imaging. Additionally, the intrinsic optical properties of Gadolinium oxide enable sharp wavelength absorptions and photostability, making it ideal for imaging purposes. By doping the Gadolinium oxide matrix with other lanthanide ions, the optical properties can be further enhanced, leading to photoluminescent materials with high Stokes shifts, sharp emission spectra, long lifetimes, minimized photobleaching, and multiphoton absorption. These unique properties make gadolinium-oxide lattices a top choice for a wide range of photoluminescence applications, including both technological and biomedical fields. 1
Synthesis Routes
Gadolinium oxide, a compound of gadolinium and oxygen, can be synthesized through various routes. One recent strategy is the sonic-chemical method, as reported by Perdigon-Lagunes et al. In this method, Gd(NO3)2 solution and tannic acid are mixed, followed by the addition of NaOH to adjust the pH. Sonication is then applied to activate clustering sites, causing Gd3+ ions to be reduced to Gd(0) atoms that bind together into metallic clusters, serving as seeds for nanoparticle formation. The nanoparticles are subsequently oxidized by the presence of oxygen and their growth is limited by freezing the reaction mixture in liquid nitrogen. The resulting nanoparticles have sizes below 7 nm, with their dimensions influenced by the pH due to the properties of tannic acid. Another approach, the solid-state technic combined with laser ablation in liquid (LAL) method, was reported by Chen et al. In this method, Gd2O3 and Tb4O7 are milled in different stoichiometric ratios, and the resulting solid targets are ablated in liquid using a microsecond laser. The obtained nanoparticles exhibit a monoclinic structure, good crystallinity, and high purity. Their average size is around 8.4 ± 0.2 nm, and they possess efficient fluorescence and MR imaging properties, making them suitable for dual-modal contrast agent applications. The sol-gel method is another commonly used technique. Niftaliev et al. synthesized gadolinium oxide nanoparticles using Gd(NO3)3 as the precursor and agar-agar as a stabilizer. The process involves hydrolysis, polycondensation, gelation, aging drying, densification, and crystallization. The resulting nanoparticles have an average size of 8-16 nm. In summary, there are multiple synthesis routes for gadolinium oxide, including the sonic-chemical method, solid-state technic, and sol-gel method. Each method offers unique advantages and can yield nanoparticles with different sizes and properties suitable for various applications. 2
Toxicity
Gadolinium oxide has been associated with various toxic effects. It is known to cause serious eye irritation upon contact. Moreover, it is highly toxic to aquatic life, posing risks of long-lasting effects on aquatic ecosystems. Additionally, gadolinium oxide has fibrogenic properties, capable of inducing tissue injury and fibrosis, leading to scarring. Due to its toxicity, proper handling and disposal measures are essential to minimize environmental impact and prevent harm to both aquatic organisms and human health. For more information on the toxicity of gadolinium oxide, references to rare earth metals and linked occupational diseases can be consulted. 3
Reference
1. Song Y, You H, Huang Y, et al. Highly uniform and monodisperse Gd(2)O(2)S:Ln(3+) (Ln = Eu, Tb) submicrospheres: solvothermal synthesis and luminescence properties. Inorg Chem. 2010;49(24):11499-11504.
2. Benita OB. An Overview of Gadolinium-Based Oxide and Oxysulfide Particles: Synthesis, Properties, and Biomedical Applications. Crystals, 2021, 20(1).
3. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 159427, Gadolinium oxide.
Related articles And Qustion
See also
Lastest Price from Gadolinium oxide manufacturers

US $40.00-3500.00/USD/KG2025-06-09
- CAS:
- 12064-62-9
- Min. Order:
- 1000USD/KG
- Purity:
- 99.5%
- Supply Ability:
- 20T per month

US $2.00/kg2025-04-21
- CAS:
- 12064-62-9
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000