Furfural: a natural precursor
Introduction
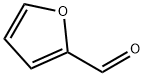
Furfural (OC4H3CHO, also called 2-formylfuran, furan-2-aldehyde, 2-furancarboxaldehyde, 2-furyl-methanal, pyromucic aldehyde, 2-furanaldehyde, 2-furancarbonal, carboxylic aldehyde, furan-2-carbaldehyde, furancarbonal, 2-furaldehyde, or 2-furfural), contains a heteroaromatic furan ring and an aldehyde functional group. Furfural was first isolated in 1832 by J.W. Döbereiner and has been industrially produced since 1922.
Synthesis
Furfural is a chemical product resulting from the hydrolysis of pentoses or other polysaccharides rich in pentoses classified into four main groups: xylans, mannans, xyloglucans, and β-glucans with subsequent dehydration of the pentoses. In a commercial process, it is produced through acid hydrolysis of biomass, usually with sulfuric acid, and its production is not economically viable through fossil-based routes.
The Quaker Oats process is the oldest commercial form of industrial furfural production. This process was created by the Quaker Oat company using oat cereal waste as raw material, which is mixed with sulfuric acid. The method consists in two steps, first the reaction zone in which the biomass reacted with a solution of sulfuric acid to convert the xylan fraction into furfural, then high vapour stream is introducing to the reactor to remove the furfural as fast as possible to avoid furfural polimerization. The vapour stream from the reactor is condensed to feed the azeotropic distillation sequences to remove the excess water and some by-products, such as methanol and acetic acid.
Use
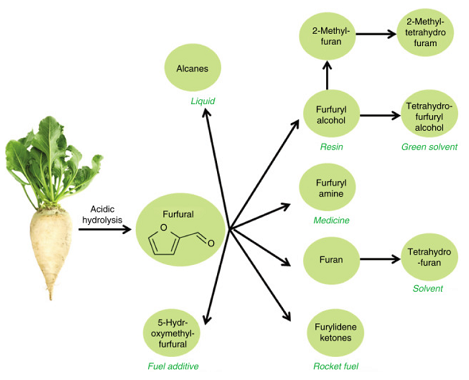
In recent years, furfural has received special attention as a potential platform to produce biofuels and biochemicals. In a study conducted by the Department of Renewable Energy of the United States, furfural was selected as one of the 30 main chemicals that can be manufactured from biomass. Industrially, it is a very versatile chemical because of its multiple applications: utilized as a raw material to produce phenol-furfural-resins. Furfural is an organic compound with a carbonyl functional group and a furan ring, which facilitates the production of chemical products such as tetrahydrofurfuryl alcohol, tetrahydrofuran, and alkanes.
Furfural and its derivatives have been extensively used in plastics, pharmaceutical and agrochemical industries. Furfural is a natural precursor to a range of furan-based chemicals and solvents such as dihydropyran, methyltetrahydrofuran, tetrahydrofuran, methylfuranfurfuryl alcohol, tetrahydrofurfuryl alcohol and furoic acid. Furfural and its derivatives have been widely applied as fungicides and nematicides, transportation fuels, gasoline additives, lubricants, resins, decolorizing agents, jet fuel blend stocks, drugs, insecticides, bio-plastics, flavour enhancers for food and drinks, rapid all-weather repair system for bomb-damaged runways and potholes and also for wood modification and book preservation.
References
[1] Furfural - an overview | ScienceDirect Topics.https://www.sciencedirect.com/topics/chemical-engineering/furfural.
Related articles And Qustion
See also
Lastest Price from Furfural manufacturers

US $10.00/KG2025-04-21
- CAS:
- 98-01-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $12.00-1.00/KG2024-12-26
- CAS:
- 98-01-1
- Min. Order:
- 800KG
- Purity:
- 99% MIN
- Supply Ability:
- 3000 kg