Fulvestrant-Palbociclib vs Letrozole-Palbociclib
Fulvestrant
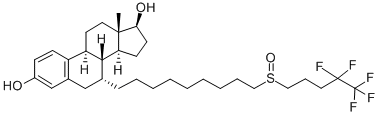
Fulvestrant is a selective estrogen receptor (ER)-downregulating antiestrogen that blocks ER transcriptional activity and is approved for ER-positive (+) breast cancer. Fulvestrant also induces the accumulation of insoluble ER and activates an unfolded protein response; proteasome inhibitors have been shown to enhance these effects in preclinical models[1].
It was first approved by the FDA in 2002 after Phase 3 trials showed that the patients on fulvestrant took longer for their cancer to get worse when compared to patients on Arimidex (anastrozole). It is approved to be used for certain types of breast cancer in women, so doctors will only prescribe it for patients if it is effective for the type of cancer.
Letrozole
Letrozole, an aromatase inhibitor that blocks estrogen synthesis by inhibiting the final step of the estrogen biosynthetic pathway, has been used in the applications of a wide range of infertility settings. Letrozole is a treatment that may be used to reduce the risk of breast cancer recurrence in postmenopausal women and also to increase the chance of ovulation in women with polycystic ovary syndrome. Side effects include hot flashes, headaches, and joint pain, which may lower bone mineral density and increase cholesterol levels[2].
Letrozole is prescribed for Female Infertility, Breast Cancer - Metastatic, Breast Cancer - Adjuvant, and Breast Cancer. Letrozole may also be used for purposes not listed in this comparison guide.
Fulvestrant vs Letrozole
Which is the optimal endocrine partner (fulvestrant vs. letrozole) for cyclin-dependent kinase 4 and 6 inhibitor palbociclib in previously untreated, endocrine-sensitive, hormone receptor–positive, ERBB2-negative advanced breast cancer?
A total of 486 women were randomized (243 to fulvestrant-palbociclib and 243 to letrozole-palbociclib). Median investigator-assessed progression-free survival was 27.9 months (95% CI, 24.2-33.1 months) in the fulvestrant-palbociclib group vs 32.8 months (95% CI, 25.8-35.9 months) in the letrozole-palbociclib group (hazard ratio, 1.13; 95% CI, 0.89-1.45; P = .32). This result was consistent across the stratification factors. No significant differences were observed in objective response rate (46.5% vs. 50.2%) and 3-year overall survival rate (79.4% vs. 77.1%) for fulvestrant-palbociclib and letrozole-palbociclib, respectively[3]. Grade 3-4 adverse events were comparable among treatment groups, and no new safety signals were identified.
References
[1] K. Kobayashi. “Abstract OT2-02-07: Fulvestrant with additional palbociclib in advanced or metastatic hormone receptor-positive HER2-negative breast cancer after progression to fulvestrant monotherapy: JBCRG- M07 (FUTURE trial).” Cancer research (2020).
[2] Ai-Min Yang. “Letrozole for Female Infertility.” Frontiers in Endocrinology (2021): 676133.
[3] Antonio Llombart-Cussac. “Fulvestrant-Palbociclib vs Letrozole-Palbociclib as Initial Therapy for Endocrine-Sensitive, Hormone Receptor-Positive, ERBB2-Negative Advanced Breast Cancer: A Randomized Clinical Trial.” JAMA Oncology 7 12 (2021): 1791–1799.
Related articles And Qustion
See also
Lastest Price from Fulvestrant manufacturers

US $0.00/kg2025-09-04
- CAS:
- 129453-61-8
- Min. Order:
- 1kg
- Purity:
- 99.0
- Supply Ability:
- 50

US $0.00-0.00/KG2025-07-01
- CAS:
- 129453-61-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1T