Fulvestrant: A Anti-Estrogen Therapy with Unique Pharmacokinetics and Low Toxicity
General Description
Fulvestrant showcases a unique pharmacokinetic profile with slow release and minimal body accumulation, achieving stable plasma concentrations for 28 days post-injection. Its high affinity for plasma proteins and liver metabolism, with primary fecal excretion, allows usage without dose adjustments in mild to moderate renal or hepatic impairments. As an anti-estrogen, fulvestrant blocks estrogen action by downregulating estrogen receptors, without partial agonist effects, offering a high safety margin demonstrated through minimal toxicity in animal studies. Administered monthly via intramuscular injection, it's contraindicated in certain populations like pregnant women. Despite its minimal drug interactions and mild adverse effects, caution is advised, especially in patients with bleeding disorders or on anticoagulants.

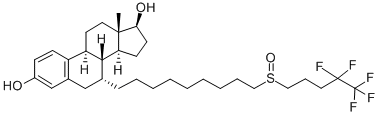
Figure 1. Fulvestrant
Pharmacokinetics
Fulvestrant's pharmacokinetic profile is characterized by its slow release from the injection site, ensuring stable plasma concentrations within a relatively narrow range for at least 28 days post-injection. This method of administration leads to minimal accumulation in the body, with exposure levels roughly doubling and reaching near steady-state after receiving 3 to 6 doses. Fulvestrant exhibits a high binding affinity to plasma proteins, with 99% of the drug being bound. It undergoes rapid metabolism in the liver, with a clearance rate exceeding 10 ml/min/kg, and is predominantly excreted through the feces, while renal elimination is minimal (less than 1%). Notably, no adjustments in dosage are necessary for patients with mild to moderate renal impairment (creatinine clearance above 30 ml/min) or those with hepatic impairments, including those with liver metastases. Similarly, elderly patients do not require dose modifications. This pharmacokinetic behavior underlines fulvestrant's efficacy and safety profile across a diverse patient population. 1
Pharmacodynamics and toxicology
Fulvestrant represents a novel class of anti-estrogen therapy, distinguished by its unique mechanism of action that leads to the downregulation of estrogen receptors (ER). It acts as a pure antagonist to estrogen, effectively blocking estrogen's proliferative actions without exhibiting any partial agonist or estrogen-like effects. Fulvestrant competes with estrogen for ER binding sites, showcasing a high affinity that rivals that of estradiol and is 100 times greater than that of tamoxifen, ensuring effective inhibition of estrogen-mediated activity. In terms of toxicology, studies on the compound ICI 182,780 (fulvestrant) have demonstrated minimal acute toxicity in animal models, with no fatalities observed at significant dosage levels administered either intramuscularly or intravenously in rats and mice. Long-term multiple-dosing studies, extending up to six months in rats and dogs, revealed no systemic toxicity, indicating a favorable safety profile. While some local irritation at the injection site was noted, it was attributed more likely to the formulation's vehicle or excipients rather than the active drug itself. Additionally, fulvestrant showed no teratogenic potential in rabbit teratology studies, further supporting its safety for therapeutic use. 2
Dosage and precautions
Fulvestrant is administered as a 50 mg/ml solution for injection, with each 5 ml containing 250 mg of fulvestrant. The solution also contains excipients such as ethanol (96%), benzyl alcohol, benzyl benzoate, and castor oil. The recommended dosage is a single slow intramuscular (IM) injection of 5 ml into the gluteus maximus every month. Alternatively, the dose can be divided into two injections of 2.5 ml each, maintaining the total monthly dose at 250 mg. It is crucial to note that fulvestrant is contraindicated in individuals with known hypersensitivity to the drug or any of its components, as well as in pregnant and lactating women. Its use is not supported in premenopausal women or patients with severe renal impairment (creatinine clearance < 30 ml/min) and/or severe hepatic impairment due to the lack of data. Caution is advised when administering fulvestrant to patients with bleeding disorders or those on anticoagulant therapy because it is delivered via IM injection. Regarding drug interactions, fulvestrant does not significantly inhibit major human cytochrome P450 isoenzymes in vitro, and there's no evidence of hepatic enzyme induction from animal studies. Additionally, no significant effect on fulvestrant metabolism by inhibitors of major P450 enzymes has been observed. The concurrent use of fulvestrant with systemic estrogens is not recommended, and there are no clinical data on its use in combination with other anticancer agents. Common adverse reactions include hot flushes, nausea, and injection site reactions, which are generally mild. Given these considerations, healthcare professionals should carefully assess patient eligibility before prescribing fulvestrant and monitor for potential side effects and drug interactions. 2
Reference
1. Curran M, Wiseman L. Fulvestrant. Drugs. 2001;61(6):807-814.
2. Cheung KL, Robertson JF. Fulvestrant. Expert Opin Investig Drugs. 2002;11(2):303-308.
You may like
Related articles And Qustion
Lastest Price from Fulvestrant manufacturers

US $0.00/kg2025-09-04
- CAS:
- 129453-61-8
- Min. Order:
- 1kg
- Purity:
- 99.0
- Supply Ability:
- 50

US $0.00-0.00/KG2025-07-01
- CAS:
- 129453-61-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1T