Ferrous Ammonium Sulfate: Applications and Preparation
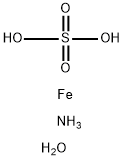
Ferrous Ammonium Sulfate also inferred as ammonium Iron (II) sulphate, or Mohr’s salt is having a chemical formula of (NH4)2Fe(SO4)2. It is a widely used laboratory double salt containing both ferrous sulfates, and ammonium sulphate. Ferrous Ammonium Sulfate is a metal sulfate, an iron molecular entity and an ammonium salt. It consists of iron (2+).

Applications of Ferrous Ammonium Sulfate
The salt is primarily used in analytical chemistry, which is preferred as a source of ferrous ions which has a long shelf life and is resistant to oxidation. The stability extends slightly to solutions reflecting the effect of pH on the ferrous/ ferric redox couple. This oxidation takes place more willingly at high pH. The ammonium ions make the solutions of Mohr's salt somewhat acidic, which slows this oxidation process. Sulfuric acid is usually supplemented to solutions to lessen oxidation to ferric iron.
Ferrous Ammonium Sulfate is used in common labs for performing a qualitative chemical analysis which is used to identify the unknown concentration of a solution. Ferrous ions of Mohr’s salt go through the hydrolysis in aqueous solution. To prevent the hydrolysis concentration, H2SO4 requires to be supplemented to the Mohr’s salt crystals when preparing its standard solution.
Why is Mohr’s salt known as double salt?
A double salt is a crystalline salt having the composition of a mixture of two simple salts but with an unlike crystal structure from either. It contains two different cations of Fe2+ and NH4+. Ferrous ammonium sulfate liquefies in water to obtain the auqo complex which has octahedral molecular geometry. Mohr’s salt possesses a molar mass of 392.21 g/mol and it appears as a blue-green crystal.
Preparation of Ferrous Ammonium Sulfate or Mohr’s salt
Mohr’s salt is prepared by dissolving an equal amount of mixture of hydrated ferrous sulphate and ammonium sulphate in water that contains sulphuric acid. This results in the solution to crystallization, therefore the solution forms light green crystals.
The equation is below:
FeSO4.7H2O + (NH4)2SO4 = FeSO4 . (NH4 )2SO4.6H2 O
Ferrous Ammonium Sulfate Toxicity
Inhalation of this salt causes irritations in the nose and throat. Intake of the chemical causes nuisance in the mouth and stomach. Toxic ammonia and oxides of nitrogen may form in fires.
You may like
See also
Lastest Price from Ferrous ammonium sulfate hexahydrate manufacturers
US $0.00/KG2025-04-21
- CAS:
- 7783-85-9
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $30.00/kg2025-04-21
- CAS:
- 7783-85-9
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100 tons