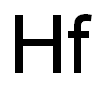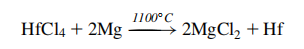Facts About Hafnium
Hafnium is a lustrous, silvery-gray transition metal. Discovered in 1923, it was the next-to-last element with stable nuclei to be added to the periodic table (the final one was rhenium in 1925). Hafnium is named after the Latin word for Copenhagen: Hafnia. The element has some very important commercial applications, including its use in the nuclear power industry, electronic equipment, ceramics, light bulbs and in the making of super-alloys.
Hafnium is rarely found free in nature, and instead is present in most zirconium minerals at a concentration of up to 5 percent. In fact, hafnium is so chemically similar to zirconium that separating the two elements is extremely difficult. Most commercial hafnium is produced as a byproduct of zirconium refining.

Hafnium is the 45th most abundant element on Earth, comprising about 3.3 parts per million (ppm) of the Earth's crust by weight, according to Chemicool. Hafnium is quite resistant to corrosion because of the formation of an oxide film on exposed surfaces. In fact, it is unaffected by water, air and all alkalis and acids except for hydrogen fluoride.
Hafnium carbide (HfC) has the highest melting point of any known two-element compound at nearly 7,034 degrees Fahrenheit (3,890 degrees Celsius), according to Jefferson Lab. The compound hafnium nitride (HfN) also has a high melting point, around 5,981 degrees F (3,305 degrees C). Among compounds of three elements, the mixed carbide of tungsten and hafnium has the single highest melting point of any known compound at 7,457 degrees F (4,125 degrees C), according to Chemistry World. Some other hafnium compounds include hafnium fluoride (HfF4) hafnium chloride (HfCl4) and hafnium oxide (HfO2).
Just the facts
Atomic number (number of protons in the nucleus): 72
Atomic symbol (on the periodic table of the elements): Hf
Atomic weight (average mass of the atom): 178.49
Density: 13.3 grams per cubic centimeter
Phase at room temperature: Solid
Melting point: 4,051 degrees Fahrenheit (2,233 degrees Celsius)
Boiling point: 8,317 degrees F (4,603 degrees C)
Number of isotopes (atoms of the same element with a different number of neutrons): 32 whose half-lives are known with mass numbers 154 to 185
Most common isotopes: Hf-174, Hf-176, Hf-177, Hf-178, Hf-179 and Hf-180.
Discovery
Hafnium's presence had been predicted decades before its discovery, according to Chemistry World. The element proved to be quite elusive, as it was nearly impossible to distinguish it chemically from the much more common zirconium.
Hafnium was still unknown when Russian chemist and inventor Dimitri Mendeleev developed the Periodic Law — a pre-modern version of the periodic table of elements — in 1869. In his work, however, Mendeleev correctly predicted that there would be an element whose properties were similar to but heavier than zirconium and titanium.
In 1911, French chemist Georges Urbain, who had already discovered the rare earth element lutetium, believed he had finally discovered missing element 72 — which he proceeded to name celtium, according to Chemicool. However, a few years later his discovery was shown to be a combination of already discovered lanthanides (the 15 metallic elements with atomic numbers 57 through 71 in the periodic table).
It was still unclear whether missing element 72 would be a transition metal or a rare earth metal since it fell at the boundary between these two types of elements in the table. The chemists who believed it would be a rare earth element conducted many fruitless searches among minerals containing rare earths, according to Chemistry World.

However, new evidence arising from both the field of chemistry and physics supported the idea that element 72 would be a transition element. For example, scientists knew that element 72 fell below titanium and zirconium in the periodic table and both of these were known transition elements. In addition, Danish physicist Niels Bohr, one of the founders of quantum theory, predicted that element 72 would be a transition metal based on his electronic configuration for the element, according to Chemistry World.
In 1921, Bohr encouraged Hungarian chemist Georg von Hevesy and Dutch physicist Dirk Costerto — two young researchers in his institute at the time — to search for element 72 in zirconium ore. Based on his quantum theory of atomic structure, Bohr knew that the new metal would have a similar chemical structure to zirconium, so there was a strong chance the two elements would be found in the same ores, according to Chemicool.
Von Hevesy and Coster took Bohr's advice and proceeded to study zirconium ore using X-ray spectroscopy. They used Bohr's theory of how electrons fill shells and subshells within atoms to predict the differences between the two elements' X-ray spectra, according to Chemical and Engineering News. This method ultimately led to the discovery of hafnium in 1923. The discovery was one of the only six then remaining gaps in the periodic table. They named the new element after Bohr's hometown of Copenhagen (Hafniain Latin).
Uses
Hafnium is remarkably corrosion-resistant and an excellent absorber of neutrons, allowing its use in nuclear submarines and nuclear reactor control rods, a critical technology used to maintain fission reactions. Control rods keep the fission chain reaction active but also prevent it from accelerating beyond control.
Hafnium is used in electronic equipment such as cathodes and capacitors, as well as in ceramics, photography flash bulbs and light bulb filaments. It is used in vacuum tubes as a getter, a substance that combines with and removes trace gases from the tubes, according to Jefferson Lab. Hafnium is commonly alloyed with other metals such as titanium, iron, niobium and tantalum. For example, heat-resistant hafnium-nobium alloys are used in aerospace applications, such as space rocket engines.
The compound hafnium carbide has the highest melting point of any compound consisting of just two elements, allowing it to be used to line high-temperature furnaces and kilns, according to Chemicool.
Who knew?
Hafnium is pyrophoric (ignites spontaneously) in powder form.
English chemist Henry Moseley was the scientist who realized that Georges Urbain's element "celtium" was not the true element located under zirconium. Unfortunately, World War I interrupted this young scientist's important research. Moseley dutifully enlisted in the Royal Engineers of the British Army and was killed by a sniper in 1915. His death led England to establish a new policy prohibiting prominent scientists from engaging in combat.
In 1925, Dutch chemists Anton Eduard van Arkel and Jan Hendrik de Boer came up with a method for producing high-purity hafnium. To do this, the scientists decomposed hafnium tetraiodide on a hot tungsten wire resulting in a crystal bar of pure hafnium, according to Chemicool. This method is called the crystal bar process.
The nuclear isomer of hafnium has long been debated as a potential weapon. In the Hafnium Controversy, scientists debate whether the element is capable of triggering a rapid release of energy.
Although zirconium is chemically very similar to hafnium, it is unlike hafnium in that it is very poor at absorbing neutrons. Therefore zirconium is used in the outer layer of fuel rods where it is important that neutrons can travel easily.
Dating Earth's layers with hafnium
In a recent study, an international team of researchers was able to confirm that Earth's first crust formed around 4.5 billion years ago, thanks to their chemical analysis of hafnium in a rare meteorite. The researchers believe the meteorite originated from the asteroid Vesta, following a large impact that sent rock fragments to Earth, according to the study press release in Science Daily. According to the researchers, meteorites are pieces of the original materials that formed all planets. For the study, they measured the ratio of the isotopes hafnium-176 and hafnium-177 in the meteorite. This gave them a starting point for Earth's composition. They compared the results with the oldest rocks on Earth, essentially confirming that a crust had already formed on the surface of the Earth around 4.5 billion years ago. Their findings are published in the Proceedings of the National Academy of Sciences (PNAS).
You may like
Related articles And Qustion
See also
Lastest Price from HAFNIUM manufacturers

US $10.00/KG2022-06-02
- CAS:
- 7440-58-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 tons