Exploring the Dual Nature of 1,2-Dimethylhydrazine Dihydrochloride: Applications and Toxicity in Chemical Synthesis
Introduction
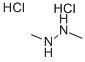
1,2-Dimethylhydrazine Dihydrochloride is a significant compound in the realm of chemistry, particularly for professionals focused on organic synthesis and medicinal chemistry. This compound is noted for its unique structure, which features two methyl groups attached to a hydrazine backbone, stabilized in the form of a dihydrochloride salt. This stabilization enhances its reactivity and handling safety, making it an invaluable reagent in various chemical transformations.
The interest in 1,2-DIMETHYLHYDRAZINE DIHYDROCHLORIDE extends beyond its chemical properties to its role in synthetic applications and research methodologies. Its versatility is reflected in its use in creating a variety of organic compounds and in studying reaction mechanisms. Furthermore, this compound serves as a pivotal element in developing drugs and other therapeutic agents, underlining its importance in advancing medical and biochemical research.
The ongoing research and development in the chemistry of 1,2-DIMETHYLHYDRAZINE DIHYDROCHLORIDE demonstrates its critical role in pushing the boundaries of science. However, this compound is not without its challenges, particularly regarding its safety and environmental impact. Thus, understanding its mechanism of action, applications, and associated risks is essential for professionals engaged in its use[1].

Figure 1 Characteristics of 1,2-DIMETHYLHYDRAZINE DIHYDROCHLORIDE
Mechanism of Action
1,2-Dimethylhydrazine Dihydrochloride operates through a mechanism that exploits its hydrazine base for various chemical reactions. The presence of two methyl groups enhances its nucleophilicity, allowing it to effectively participate in electrophilic substitution reactions. This ability makes it a versatile reagent in organic synthesis, where it can introduce nitrogen-containing functional groups into organic frameworks.
The chemical reactivity of 1,2-DIMETHYLHYDRAZINE DIHYDROCHLORIDE also involves its decomposition under certain conditions to form highly reactive free radicals. These radicals can initiate polymerization reactions or engage in radical-mediated synthesis pathways, which are crucial for creating complex molecular architectures in pharmaceuticals and high-performance materials.
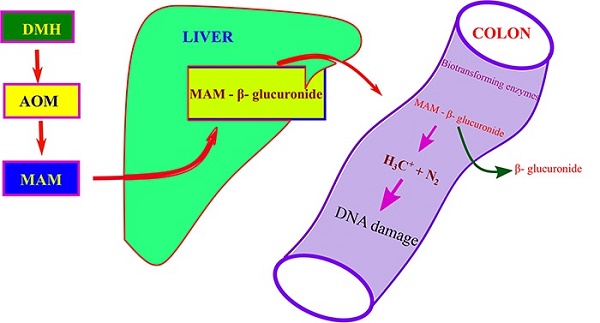
Moreover, in biological systems, 1,2-DIMETHYLHYDRAZINE DIHYDROCHLORIDE has been studied for its interaction with biomolecules. The compound's ability to release nitrogen radicals can lead to DNA alkylation, a mechanism that has profound implications in both therapeutic applications and toxicological impacts. Understanding these interactions at the molecular level is vital for chemists and biochemists aiming to manipulate their properties for desired outcomes in drug design and toxicology studies.
Applications
1,2-Dimethylhydrazine Dihydrochloride finds its utility in a myriad of applications across various industries, most notably in pharmaceuticals and specialty chemicals. In the pharmaceutical sector, this compound is instrumental in synthesizing active pharmaceutical ingredients (APIs) that require complex nitrogen-based structures. Its ability to introduce nitrogen groups efficiently makes it invaluable in building molecular frameworks found in many therapeutic agents, including antiviral drugs, antidepressants, and anticancer compounds.
Beyond medicine, 1,2-DIMETHYLHYDRAZINE DIHYDROCHLORIDE plays a crucial role in agricultural chemistry. It is used in the synthesis of pesticides and herbicides, where its chemical properties help in constructing compounds that are effective at controlling pests and weeds at molecular levels. This application is particularly important for developing more environmentally friendly agricultural products that offer high efficacy while reducing the ecological footprint.
Additionally, research laboratories utilize 1,2-DIMETHYLHYDRAZINE DIHYDROCHLORIDE in method development for chemical analysis and environmental testing. The compound's predictable reactivity and the ability to form stable products under controlled conditions make it an excellent candidate for developing standard testing procedures, which are vital for ensuring compliance with environmental and safety regulations[2].
Toxicity
Despite its valuable applications, 1,2-Dimethylhydrazine Dihydrochloride poses significant health risks that must be carefully managed. The primary concern is its potent toxicity, particularly its carcinogenic potential. Studies have shown that exposure to 1,2-DIMETHYLHYDRAZINE DIHYDROCHLORIDE can lead to serious health issues, including liver and lung cancer, as it metabolizes in the body to form mutagenic and carcinogenic by-products.
Occupational exposure is a significant risk factor for workers in industries using this compound. Inhalation of dust or vapors and skin contact are the most common exposure routes, necessitating stringent control measures in workplaces. These include the use of protective equipment, proper ventilation systems, and rigorous training on handling procedures.
References
[1]Goldin B R, Gorbach S L. Effect of Lactobacillus acidophilus dietary supplements on 1, 2-dimethylhydrazine dihydrochloride-induced intestinal cancer in rats[J]. Journal of the National Cancer Institute, 1980, 64(2): 263-265.
[2]Toth B. Tumorigenesis studies with 1, 2-dimethylhydrazine dihydrochloride, hydrazine sulfate, and isonicotinic acid in golden hamsters[J]. Cancer Research, 1972, 32(4): 804-807.
Related articles And Qustion
Lastest Price from 1,2-Dimethylhydrazine Dihydrochloride manufacturers
US $0.00-0.00/kg2023-12-04
- CAS:
- 306-37-6
- Min. Order:
- 0.1kg
- Purity:
- 98%
- Supply Ability:
- 100kg