Ethyl 4-Hydroxy-3-Methoxycinnamate: Sources, UV Filter Role & Photodynamics
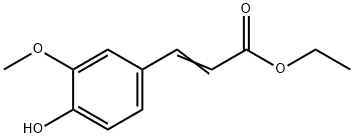
Ethyl 4-hydroxy-3-methoxycinnamate is a natural organic compound with important biological activities, its chemical name is ethyl 3 - methoxy - 4 - hydroxy cinnamate, molecular formula C₁₂H₁₄O₄, molecular weight of 222.2. It is a functional ingredient that has attracted much attention in recent years. In terms of sources, there are two main ways to obtain Ethyl 4-hydroxy-3-methoxycinnamate. On the one hand, it is widely found in natural plants, such as Angelica sinensis, Rhizoma Ligustici Chuanxiong, Shengma and other traditional Chinese medicines, as well as wheat bran, rice bran and other cereal by-products, is an important phenolic metabolite in the body of plants; on the other hand, with the increase in the demand for industrialized production, the market is more often prepared through the chemical synthesis method, ferulic acid and ethanol as the raw material, generated by the esterification reaction under the action of catalysts, which has lower cost, stable yield, and can meet the requirements for the functional components of food and cosmetics in recent years. Ethyl 4-hydroxy-3-methoxycinnamate exhibits unique characteristics in terms of physicochemical properties. It is usually white to light yellow crystalline powder, no obvious odor, melting point between 60-62 ℃, soluble in ethanol, ether, chloroform and other organic solvents, insoluble in water.

The sunscreen filter Ethyl 4-hydroxy-3-methoxycinnamate
Many living organisms have an intrinsic need for protection against ultraviolet (UV) radiation emitted by the Sun. While the solar spectrum peaks in the visible range (700–400 nm), a significant amount of UV-A and UV-B radiation (400–315 nm and 315–280 nm, respectively) reaches the surface of the Earth; on a sunny day, approximately 220 μW cm−2 globally. Ethyl 4-hydroxy-3-methoxycinnamate (ethyl ferulate, EF), was chosen for the present studies due to its use in commercial sunscreens as a UV filter and antioxidant. Similar to all active sunscreen ingredients, EF is used in these products as part of a combination of radiation filtering species in order to achieve optimum filtering efficiency across the entire UV-A and UV-B range. The broad absorption of EF in the UV region, justifies the presence of Ethyl 4-hydroxy-3-methoxycinnamate in sunscreen formulas. The research presented here explores the excited state photodynamics of EF, building on previous studies of sunscreen molecules published in recent years. EF presents itself as an extremely relevant system to be studied, not only due to its practical relevance as a sunscreen filter molecule, but also due to the insight provided into how increasing molecular complexity, in particular the addition of a carbonyl moiety (through the ester group functionality), influences the photochemistry of EF and other structurally related sunscreen molecules.[1]
We believe this decay pathway is also absent in Ethyl 4-hydroxy-3-methoxycinnamate for the same reasons; the presence of two weak KE energy features centred at ∼2500 and ∼12 500 cm−1 are assigned to multiphoton processes and/or statistical unimolecular decay. By way of contrast, the excited state dynamics of EF are notably different to MVP and ConA, as they now contain three components.One may surmise from the present measurements that, if the long lived state of Ethyl 4-hydroxy-3-methoxycinnamate is particularly reactive, be it through emission of radiation or photofragmentation/photocyclisation, it may interact with other sunscreen components or even DNA possibly leading to detrimental consequences. These measurements also suggest that further pump-probe studies on Ethyl 4-hydroxy-3-methoxycinnamate prepared in a more realistic environment, such as microsolvation and/or solution phase studies, are imperative in assessing how external perturbations influence the electronic structure which, in turn, may impact the dynamics. Importantly however, we believe that the present studies serve to highlight the significance of garnering a molecular level understanding of the underlying mechanisms of photoprotection in a commercial sunscreen constituent in the absence of any external perturbations. We hope that such a study will lay further foundations for future research, with potentially far reaching repercussions in developing safer and more efficient UV radiation blocking products.
References
[1]Rodrigues ND, Staniforth M, Young JD, Peperstraete Y, Cole-Filipiak NC, Gord JR, Walsh PS, Hewett DM, Zwier TS, Stavros VG. Towards elucidating the photochemistry of the sunscreen filter ethyl ferulate using time-resolved gas-phase spectroscopy. Faraday Discuss. 2016 Dec 16;194:709-729. doi: 10.1039/c6fd00079g. PMID: 27711798.
You may like
See also
Lastest Price from Ethyl 4-hydroxy-3-methoxycinnamate manufacturers

US $0.00/KG2025-04-21
- CAS:
- 4046-02-0
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $500.00-300.00/kg2025-04-21
- CAS:
- 4046-02-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000