Ertapenem: Antimicrobial Activity, Susceptibility, Administration and Dosage, Clinical Uses etc.
Ertapenem (formerly MK-0826; Merck) is a once-daily parenteral b-lactam antibiotic licensed in the USA in November 2001 and in Europe in April 2002. Generally, ertapenem has substantial activity against Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs) and AmpC beta-lactamases (Hammond, 2004; Wexler, 2004).
Carbapenems have been subdivided into two groups depending on their activity against non-fermentative Gram-negative bacteria (Shah and Isaacs, 2003). Ertapenem is a group 1 carbapenem in that it has restricted activity against Pseudomonas aeruginosa and Acinetobacter species (Hammond, 2004; Wexler, 2004). In contrast, imipenem (Chapter 36, Imipenem), meropenem, and doripenem (see Chapter 38, Doripenem) would be regarded as group 2 carbapenems.
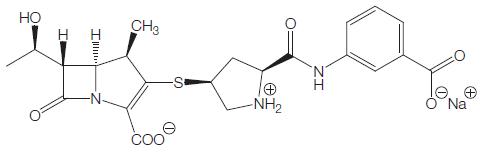
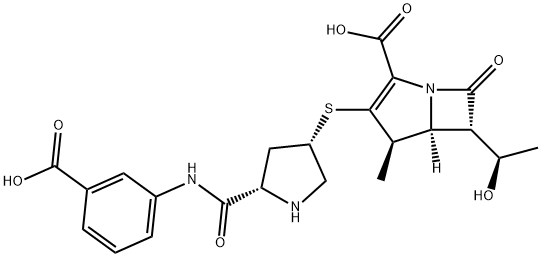
Figure 39.1 Chemical structure of ertapenem.
ANTIMICROBIAL ACTIVITY
a. Routine susceptibility
The interpretative criteria for in vitro ertapenem susceptibility testing are summarized in Table 39.1, whereas the in vitro activity of ertapenem against common pathogens is summarized in Tables 39.2, 39.3, and 39.4.
Gram-positive aerobic bacteria
Ertapenem MICs for Gram-positive bacteria are generally higher than those of imipenem and generally similar to those of meropenem. Ertapenem offers good activity against the most common Grampositive pathogens (see Table 39.2). Notable exceptions include enterococci, methicillin-resistant Staphylococcus aureus (MRSA), oxacillin-resistant coagulase-negative staphylococci, S. haemolyticus, and some Streptococcus viridans (Fuchs et al., 1999; Fuchs et al., 2000; Pankuch et al., 2002; Rolston et al., 2002; Hammond, 2004).
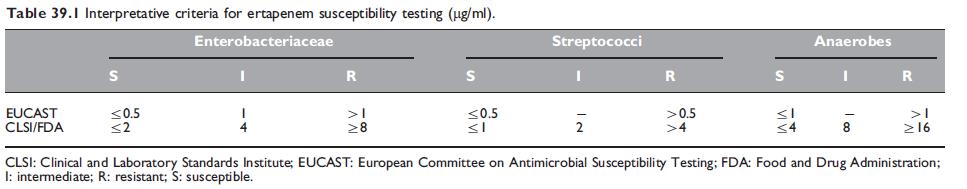
Table 39.1 Interpretative criteria for ertapenem susceptibility testing (mg/ml).

Table 39.2 In vitro activity of ertapenem against Gram-positive aerobic bacteria.

Table 39.3 In vitro activity of ertapenem against Gram-negative aerobic bacteria.
Gram-negative aerobic bacteria
The in vitro activity of ertapenem against common Gram-negative aerobic pathogens is summarized in Table 39.3. Neisseria meningitidis (Fuchs et al., 2001; Livermore et al., 2001a) is susceptible to ertapenem (MICs o0.016 mg/ml). N. gonorrhoeae is also susceptible to ertapenem. To evaluate its possible utility, Livermore et al. (2004) tested ertapenem’s microbiologic activity against 652 gonococci collected in 26 clinics in England and Wales for a three-month period (June– August 2003, inclusive). Ertapenem MICs for the 652 isolates clustered from 0.002 to 0.06 mg/ml, with fewer than 10% of values outside this range. These findings are positive for ertapenem and suggest that in vivo efficacy is likely.
Anaerobic bacteria
Most anaerobic bacteria are susceptible to ertapenem (see Table 39.4). Anaerobic Gram-positive cocci such as Peptostreptococcus are susceptible to ertapenem (MIC 0.2–1 mg/ml) (Goldstein et al., 2000; Wexler et al., 2000; Goldestein et al., 2001; Aldridge, 2002; Hoellman et al., 2002; Pelak et al., 2002). Clostridium spp. susceptibility to ertapenem depends on the individual species (Goldstein et al., 2000; Wexler et al., 2000; Hoellman et al., 2002; Pelak et al., 2002). A large study surveying 1001 anaerobes from 17 countries found that MICs90 were species-specific for Clostridium, ranging from 0.06 mg/ml for Clostridium perfringens, to 4mg/ml for C. clostridioforme (Goldstein et al., 2000). Among all these studies, C. difficile was found the most resistant, with MICs ranging from 1 to 8 mg/ml. Lactobacillus spp. may also be resistant to ertapenem (Goldstein et al., 2000; Hoellman et al., 2002). In the study of Goldstein et al. (2000), the Lactobacillus spp. MIC90 to ertapenem was W32 mg/ml.

Table 39.4 In vitro activity of ertapenem against anaerobic bacteria.
b. Emerging resistance and cross-resistance
The best-described mechanisms of carbapenem resistance include changes in outer membrane proteins, multidrug efflux pumps, and potent beta-lactamases that hydrolyze carbapenems.
Ertapenem’s lack of activity against MRSA and E. faecium, and its reduced activity against penicillin-resistant streptococci, are mediated by Penicillin-binding protein (PBP) 2a, a synthetic bacterial cell wall penicillin-binding protein with a low affinity of binding to beta-lactams that is encoded by mecA (Chambers, 1995; Katayama et al., 2004). In Pseudomonas species, both porins (loss of OprD) and efflux pumps have been implicated in carbapenem resistance (Ochs et al., 2000). Presumably, this mixture of ‘‘impermeability’’ and efflux is responsible for the lack of activity of ertapenem against P. aeruginosa.
c. In vitro synergy and antagonism
Synergistic combinations with ertapenem plus other antibiotics have been tested (Burgess and Nathisuwana, 2002; Frank, 2004; Marcheses et al., 2004; Jacqueline et al., 2006). In the study of Burgess and Nathisuwana (2002), ertapenem plus ciprofloxacin or gentamicin against S. aureus, coagulase-negative staphylococci, E. coli, and Klebsiella had a synergistic, additive, or indifferent effect, but never antagonistic. In the study of Jacqueline et al. (2006), in vitro and in vivo activity of linezolid combined with ertapenem was a highly synergistic combination against MRSA. Frank (2004) found that the combination effect of ertapenem with aminoglycosides against Gram-positive and Gram-negative community-acquired pathogens was always synergistic or additive. Marchese et al. (2004) evaluated the time-kill of the in vitro activity of ertapenem plus clarithromycin, levofloxacin, rifampicin, and vancomycin against S. pneumoniae, finding a high bactericidal activity of combinations in the first 24 hours of experimentation.
MECHANISM OF DRUG ACTION
Ertapenem rapidly penetrates the bacterial membrane and inhibits cell wall synthesis by way of binding to PBPs. Ertapenem binds most strongly to PBP-2 of E. coli, then PBP-3, and also has good affinity for PBP-1a, -1b, 4, and 5. By contrast, imipenem binds primarily to PBP-2, then -1a and -1b, and has only weak affinity for PBP-3. Ertapenem and meropenem saturate their primary PBP targets in E. coli at lower concentrations than does imipenem, probably explaining why they have lower MICs. Permeation assays have not been reported, but as a larger and more negatively charged molecule, ertapenem is likely to permeate Gram-negative bacteria more slowly than meropenem. Inactivation of PBP-1a and -1b achieves rapid bactericidal action, without the previous filamentation that occurs with agents, such as third-generation cephalosporins, which bind primarily to PBP-3. This means that the carbapenems allow a smaller increase in biomass before cell lysis, potentially minimizing endotoxin release, and organism inflammatory damage (Livermore et al., 2003; Gobernado and Acun˜a, 2007).
MODE OF DRUG ADMINISTRATION AND DOSAGE
a. Adults
The dose of ertapenem is 1 g given once daily by the intravenous or intramuscular route. Concerns have been expressed as to whether this is the most appropriate dosing regimen for critically ill individuals, for whom the optimal dosing regimen has not yet been established.
b. Newborn infants and children
Ertapenem use in a pediatric population has been described in children from three months to 12 years of age (FDA, 2005; Yellin et al., 2007; Arguedas et al., 2009). Ertapenem is not recommended for use in children below three months of age owing to a lack of data on safety and efficacy. The dose of ertapenem in children is 15 mg/kg given twice daily (not to exceed 1 g/day) by the intravenous route. No data are available to make recommendations on dosing in premature neonates.
TOXICITY
Data from early phase clinical pharmacology studies showed no dose relationship for clinical or laboratory adverse events (AEs) associated with i.v. or i.m. ertapenem at doses of o1–3 g and no clinically significant prolongation of the QTc interval following i.v. administration of 2-g ertapenem. In addition, no dose relationship was demonstrated in the phase II clinical trials.
The incidence of most clinical and laboratory AEs was similar between ertapenem 1 g and the comparator agents, piperacillin– tazobactam and ceftriaxone (Table 39.7). The rates of serious AEs and discontinuations because an AE associated with ertapenem were also low and generally similar to those due to the comparators (Livermore et al., 2003; Teppler et al., 2004).
a. Hypersensitivity reactions and rash
Ertapenem is contraindicated in patients with known hypersensitivity to any component of this product or to other carbapenems, or in patients who have demonstrated anaphylactic reactions to other beta-lactam antibiotics. There have been reports of individuals with a history of penicillin hypersensitivity who have experienced severe hypersensitivity reactions when treated with another beta-lactam. Owing to the use of lidocaine as a dilutent, ertapenem administered intramuscularly is contraindicated in patients with a known hypersensitivity to local anesthetics of the amide type.
b. Gastrointestinal side-effects
Diarrhea was reported in 1.7–7% of patients treated with ertapenem, nausea in 0.8–7.0%, and vomiting in 0.9%. None of these rates was significantly different from those found for the comparator agents. C. difficile-associated colitis has been reported following ertapenem use (Graham et al., 2002; Solomkin et al., 2003). In a randomized trial of prophylaxis against infection following colorectal surgery, the overall incidence of C. difficile infection was 1.7% in the ertapenem group and 0.6% in the cefotetan group (p=0.22).
c. Effects on gut flora
In healthy volunteers, ertapenem induced a marked decrease in the anaerobic microflora (4-log-unit decreases in lactobacilli, bifidobacteria, clostridia, and Bacteroides) and E. coli, whereas the number of enterococci increased (4 log units). A slight overgrowth of yeasts was observed. In all cases the microflora returned to normal levels on days 21–35 (Pletz et al., 2004). In comparative evaluations, ertapenem was less likely to select for ertapenem-resistant organisms than piperacillin– tazobactam was to select for piperacillin–tazobactam-resistant organisms (DeNubile, 2005).

Table 39.7 Incidence (%) of clinical adverse events (AEs) occurring with a frequency of Z1% during study therapy (parenteral plus optional oral)
and for 14 days thereafter in patients in the ertapenem (ETP), piperacillin–tazobactam (P/T) or ceftriaxone (CRO) treatment groups of the phase II
and III trials (Teppler et al., 2004).
d. Hematologic side-effects
Drug-related neutropenia is infrequent and, when it occurred, almost always mild and transient. In phase III trials, neutropenia was rarely a cause for discontinuation of study therapy (Teppler et al., 2004). Neutropenia is a known but uncommon complication of therapy with any b-lactam agents (Olaison and Alestig, 1990).
e. Altered liver function tests
The most common laboratory AE associated with ertapenem and both comparator agents was mild-to-moderate elevation of aminotransferase levels. Increased alanine aminotransferase levels were seen in 3.3–9.0% of patients, increased aspartate aminotransferase in 2.1– 8.0%, and increased alkaline phosphotransferase in 1.4–7.0%. These increases tended to be transient, returning to normal or near-normal levels by the time follow-up testing was performed, and without clinical consequence.
f. Local effects: Pain and phblebitis
Between 3.2% and 15.3% of patients experienced at least one local reaction at the infusion site, although three-quarters of these amounted to no more than local erythema.
g. Other side-effects
Seizures have been associated with meropenem and imipenem. In the clinical trials reported, seizures in patients treated with ertapenem 1 g were rare, irrespective of drug relationship (0.5% during study therapy plus the 14-day follow-up period, vs 0.3% for piperacillin–tazobactam) and almost all patients who experienced a seizure had underlying central nervous system disease or a known seizure disorder. The incidence during parenteral therapy of seizures considered to be drug related in patients who received ertapenem therapy (0.2%) was similar to the incidence in patients in these trials who were treated with piperacillin–tazobactam (0.3%) (Teppler et al., 2004).
h. Risk in pregnancy
In mice and rats given i.v. doses of up to 700 mg/kg/day (for mice, approximately three times the recommended human dose of 1 g based on body surface area and for rats, approximately 1.2 times the human exposure at the recommended dose of 1 g based on plasma AUCs), there was no evidence of developmental toxicity as assessed by external, visceral, and skeletal examination of the fetuses. However, in mice given 700 mg/kg/day, slight decreases in average fetal weights and an associated decrease in the average number of ossified sacrocaudal vertebrae were observed. Ertapenem crosses the placental barrier in rats. There are, however, no adequate and well-controlled studies in pregnant women. Ertapenem is listed as pregnancy category B.
CLINICAL USES OF THE DRUG
The spectrum of ertapenem, with activity against Enterobacteriaceae and anaerobes, lends itself to the treatment of polymicrobial infections, particularly where Acinetobacter spp. and P. aeruginosa are uncommon pathogens (Wexler, 2004). Major clinical trials in these settings are outlined in Tables 39.8, 39.9, and 39.10. As with most drug evaluations, these were powered to demonstrate equivalence, not superiority. Ceftriaxone, with or without metronidazole, ticarcillin–clavulanic acid, or piperacillin–tazobactam served as the comparators. All the trials were randomized, multicenter, and doubleblinded.
a. Intra-abdominal infections
Six trials in intra-abdominal infections have been published (Table 39.8) (Yellin et al., 2002; Solomkin et al., 2003; Navarro et al., 2005; Dela Pena et al., 2006; Namias et al., 2007; Basoli et al., 2008). There is also a trial considering mixed infections in children (Yellin et al., 2007), in whom the treatment with ertapenem is compared with ticarcillin–clavulanic acid.
The study of Solomkin et al. (2003) was a prospective randomized controlled double-blind trial conducted to compare the safety and efficacy of ertapenem with piperacillin–tazobactam as therapy following adequate surgical management of complicated intra-abdominal infections. The modified intent-to-treat population included 633 patients, with 396 meeting all criteria for the evaluable population. Of 203 microbiologically evaluable patients, 176 treated with ertapenem (86.7%) were cured, as were 157 of the 193 (81.2%) treated with piperacillin–tazobactam. This difference was not statistically significant.

Table 39.8 Randomized clinical trials of ertapenem in patients with intra-abdominal infections.

Table 39.9 Randomized clinical trials of ertapenem in patients with community-acquired pneumonia, pneumonia acquired in skilled-care facilities or in hospitals outside the intensive care unit.

Table 39.10 Randomized clinical trials of ertapenem in patients with various infections.
b. Community-acquired pneumonia
Three trials in severe community-acquired pneumonia (CAP) requiring hospitalization have been published in adults, comparing ertapenem 1 g once daily with ceftriaxone 1 g once daily (Table 39.9) (Legua et al., 2002; Ortiz-Ruiz et al., 2002; Vetter et al., 2002).
Additionally, there is a trial in children with a variety of infections (including CAP) who were treated with ertapenem 1 g i.v. once daily versus ceftriaxone 1 g i.v. once daily with similar clinical results in both groups (Arguedas et al., 2009).
c. Hospital-acquired pneumonia
Yakovlev et al. (2006) compared the efficacy and safety of twice-daily cefepime with optional metronidazole to once-daily ertapenem as initial therapy. After 3 days of parenteral therapy, participants demonstrating clinical improvement could be switched to oral ciprofloxacin or another appropriate oral agent. In this study population, ertapenem was as well tolerated and efficacious as cefepime for the initial treatment of pneumonia acquired in skilled-care facilities or in hospital environments outside the intensive care unit (ICU).
d. Acute pelvic infections
Acute soft-tissue pelvic infections in women include several diagnoses that may be categorized as infections related to delivery and those which occur after gynecologic surgery. Although these procedures are often preceded and/or followed by antimicrobial prophylaxis (e.g. cesarian section), the rate of infection may be as high as 20%. Acute pelvic infections are usually polymicrobial.
e. Skin and soft-tissue infections, including diabetic foot infection
There are two trials comparing ertapenem efficacy to piperacillin– tazobactam for the treatment of diabetic foot infection (Table 39.10) (Graham et al., 2002; Lipsky et al., 2005).
f. Urinary tract infections
Ertapenem is indicated for the treatment of severe, complicated urinary tract infections (cUTIs), particularly in cases at risk of having resistant Gram-negative pathogens and as part of directed therapy in cases with isolates demonstrating the presence of ESBLs and/or fluoroquinolone-resistant microorganisms (see Table 39.10).
g. Colorectal surgery prophylaxis
Several characteristics of ertapenem allow its use as a potential preoperative antimicrobial agent in elective colorectal surgery: it can be given by rapid intravenous administration; it has appropriate coverage against potential pathogens; it has a long half-life (so it does not require a second administration during most surgeries); and it has a safety profile similar to that of other commonly used antibiotics.
However, the use of carbapenems as prophylaxis is generally not considered to be appropriate because of their very broad spectrum of activity.
References
Arguedas A, Cespedes J, Botet FA et al. for the Protocol 036 Study Group
(2009). Safety and tolerability of ertapenem versus ceftriaxone in a doubleblind
study performed in children with complicated urinary tract infection,
community-acquired pneumonia or skin and soft-tissue infection. Int J
Antimicrob Agents 33: 163.
Baquero F, Cercenado E, Cisterna R et al. (2006). Patrones de sensibilidad a
antimicrobianos de Enterobacteriaceae causantes de infecciones
intraabdominales en Espan˜a: Resultados del estudio SMART 2003. Rev Esp
Quimioterap 19: 51.
Basoli A, Chirletti P, Cirino E et al., Italian Study Group (2008). A prospective,
double-blind, multicenter, randomized trial comparing ertapenem 3 vs Z5
days in community-acquired intraabdominal infection. J Gastrointest Surg 12:
592.
Chen M, Nafziger AN, Drusano GL, Bertino JS (2006). Comparative
pharmacokinetics and pharmacodynamic target attainment of ertapenem in
normal-weight, obese, and extremely obese adults. Antimicrob Agents
Chemother 50: 1222.
Dela Pena A, Asperger W, Kockerling F et al. for the Optimizing Intra-
Abdominal Surgery with Invanz (OASIS)-I Study Group (2006). Efficacy
and safety of ertapenem versus piperacillin-tazobactam for the treatment of
intra-abdominal infections requiring surgical intervention. J Gastro Surg 10:
567.
FDA (2005). Food and Drug Administration. Executive summary. Division of
anti-infective and ophthalmology products. Summary of clinical review of studies submitted in response to a pediatric written request. Available from:
www.fda.gov/Cder/foi/esum/2005/021337s018_Invanz_Clinical_BP CA.pdf.
Graham D, Lucasti C, Malafaia O et al., the Ertapenem Complicated Skin and
Skin Structure Infections Study Group (2002). Ertapenem once daily versus
piperacillin-tazobactam 4 times per day for treatment of complicated skin
and skin-structure infections in adults: results of a prospective, randomized,
double-blind multicenter study. Clin Infect Dis 34: 1460.
Hammond ML (2004). Ertapenem: A group 1 carbapenem with distinct
antibacterial and pharmacological properties. J Antimicrob Chemother 53
(Suppl. S2): ii7.
Itani K, Wilson SE, Awad S et al. (2006). Ertapenem versus cefotetan
prophylaxis in elective colorectal surgery. N Engl J Med 355: 2640.
Jacoby GA, Mills DM, Chow N (2004). Role of b-lactamases and porins in
resistance to ertapenem and other b-lactams in Klebsiella pneumoniae.
Antimicrob Agents Chemother 48: 3203.
Jacqueline C, Caillon J, Grossi O et al. (2006). In vitro and in vivo assessment of
linezolid combined with ertapenem: a highly synergistic combination against
methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 50:
2547.
See also
Lastest Price from Ertapenem manufacturers

US $0.00/KG2025-04-22
- CAS:
- 153832-46-3
- Min. Order:
- 1KG
- Purity:
- 0.99
- Supply Ability:
- 1000KG

US $9.90/KG2025-04-21
- CAS:
- 153832-46-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt