Ergosterol as an Immunoactive Fungal Molecule
Basic Introduction
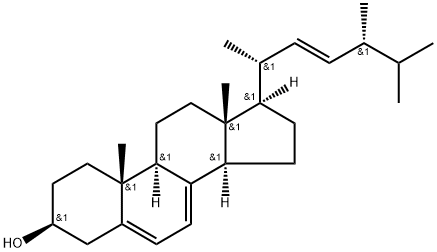
Ergosterol, a white crystalline organic solid of the molecular formula C28H44O belonging to the steroid family. It is found only in fungi (e.g, Saccharomyces and other yeasts and Claviceps purpurea, the cause of ergot, a fungal disease of cereal grasses) and is chemically related to cholesterol. Ergosterol is converted by ultraviolet irradiation into ergocalciferol, or vitamin D2, a nutritional factor that promotes proper bone development in humans and other mammals. Its relationship to vitamin D was established in 1927, when it was shown that an irradiated sample of ergosterol could be used to alleviate rickets, a deficiency disease of bone caused by lack of vitamin D in the diet. Commercially, ergosterol is produced from yeast and then converted into vitamin D2, which is used as a food supplement.
Chemical Synthesis
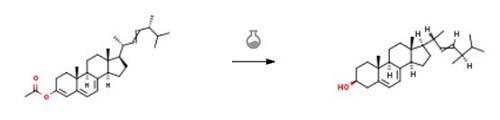
Add 180g of absolute ethanol, 180g of DCM, 6g of anhydrous calcium chloride to the reaction flask, and stir to dissolve the anhydrous calcium chloride; add 60g of intermediate 5O, and stir. Control the temperature in the kettle to about 20°C, start adding 6g of sodium borohydride in batches, and complete the addition within 20 minutes. React at 30-35 degrees for 2-3 hours. TLC tracked until there was no raw material, and the reaction was not complete and extended the time until the reaction was complete. The developing solvent PE:EA:DCM 5:1:2. After the reaction is over, the temperature is controlled, and the standby solution is slowly added dropwise. 60mL 3% hydrochloric acid. Concentrate DCM and ethanol, add 200 mL DCM, and adjust to weak acidity with 3% hydrochloric acid. Separate the organic phase, wash with aqueous sodium bicarbonate solution, concentrate the organic phase under reduced pressure, add ethanol and continue to concentrate until there is no DCM, continue to concentrate until thick, stir and crystallize at 0°C for 1-2 hours. The precipitated solid was collected by filtration, and dried at 45-50°C in the dark to obtain 45 g of solid. The yield is about 75%, and the purity is 96%.[1]
Industrial and Commercial Aspects of Ergosterol
Commercial ergostero of 90–100% purity is isolated exclusively from plant sources (usually yeast fermentation) and often contains up to 5 wt% of 5,6-dihydroergosterol. Many of the companies which began producing ergosterol early on were involved in yeast fermentation, such as breweries. The product was obtained through isolation of total sterol content from the fermentation product and the subsequent separation of the provitamin from the other sterols. This isolation of the sterol fraction involves extraction of the total fat component, saponification, and extraction of the unsaponifiable (sterol containing) portion, usually with an ether. Another method is the saponification of the total fermentation material, followed by isolation of the nonsaponifiable fraction. Separation of the sterols from the unsaponifiable fraction was done by crystallization using a suitable solvent, e.g., acetone or alcohol. The ergosterol was then recrystallized from ethylene dichloride, alone or mixed with methanol. Ergosterol is particularly difficult to remove from yeast by simple extraction, usually resulting in only ca. 25% recovery. Ergosterol procedures were developed in which digestion with hot alkalies or with amines was used. Variations of the isolation procedure have been developed; after saponification, for example, the fatty acids may be precipitated as calcium salts, which absorb the sterols. The latter are then recovered from the dried precipitate by solvent extraction. More recently new methods have been developed in China. Most of the vitamin D2 made today comes from factories in South China where an abundant source of molasses from sugar cane or cassava is available for use in the fermentation media. In this medium, molasses represents the carbon source, yeast and urea are the nitrogen source and potassium phosphate is the phosphorus source. A variety of mineral salts including magnesium sulfate are also present. The 38-hour fed batch process controls the substrate concentration by maintaining the sugar level at 0.3–0.5%. The yield is in the range of 42 grams/liter of yeast cells containing 3–4 grams of ergosterol/100 grams of yeast cells. The ergosterol is isolated by saponification of the yeast fermentate at 130°C with hot alkalis or amines, and removal of the protein and nucleic acid fraction by methanol extraction. The solvent is concentrated and the product is crystallized and isolated by filtration. Ethanol, acetone, and dichloroethylene or mixtures thereof are used to recrystallize the product. Annual production in China is over 10 metric tons by this method.[2]
In North China where there is a preponderance of antibiotic fermentation facilities, ergosterol has also been produced using extraction from hyphae of penicillin production. Wet penicillin hypha contains 0.76% of ergosterol. Saponification is accomplished with 2.5 mol/L NaOH, 25% methanol solution at 100°C for 180 minutes. Extraction using ether or petroleum ether results in ergosterol with a yield of about 0.71% (4 g ergosterol/kg hyphae with a purity of 85–90%). Aspergillus niger for citric acid fermentation and Rhizopus for lactic acid fermentation are also organisms that serve as sources for ergosterol manufacture.
Ergosterol as an Immunoactive Fungal Molecule
Inflammasome-mediated activation results in a proinflammatory form of programmed cell death known as pyroptosis. As reviewed by Man and colleagues, pyroptosis is a form of necrotic and inflammatory programmed cell death induced by inflammatory caspases. The caspases required for activation of pyroptosis include human and mouse caspase-1, human caspase-4 and caspase-5, and mouse caspase-11. Caspases mediate cleavage and activation of the pore-forming effector protein gasdermin D. Pore-mediated cell rupture results in the extracellular release of proinflammatory cytokines and alarmins and in danger-associated molecular patterns, which participate in the control of bacterial, viral, fungal, and protozoan infections. Results obtained from experimental models by the use of mice deficient in caspase-1 and/or caspase-11 and infected with Aspergillus fumigatus, Paracoccidioides brasiliensis, or Candida albicans have suggested that organ damage, increased fungal dissemination, and reduced host survival might be related to pyroptosis. Until recently, however, yeast-triggered pyroptosis has been observed only in vitro. In their recent study, Koselny and colleagues showed that pyroptosis occurs in the initial stages of murine kidney infection.[3]
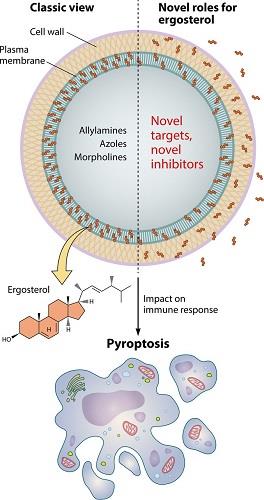
A previous study demonstrated that C. albicans-induced macrophage pyroptosis is driven by fungal cell-wall remodeling and exposure of glycosylated proteins in response to the macrophage phagosome. In the study by Koselny and colleagues, cell surface localization and/or total levels of ergosterol correlated with the ability of S. cerevisiae, C. albicans, and C. neoformans to trigger pyroptosis. By using a filamentous strain of S. cerevisiae (Σ1278b) that induces inflammasome activation and a nonfilamentous strain (S288c) that does not, they identified a set of genes involved in ergosterol and membrane homeostasis as candidates to participate in the induction of pyroptosis-related macrophage lysis. Several genes required for macrophage lysis were also required for filamentation. This suggests that fungal ergosterol may be the trigger for this mammalian cell process.
Such a relationship between ergosterol and pyroptosis has been implied by previous reports.The key transcriptional regulator of ergosterol biosynthesis, Upc2, was required for C. albicans-induced pyroptosis, and functional deactivation of Upc2 function led to a reduced amount of cellular ergosterol. In the work from Koselny and colleagues, the first experimental evidence that ergosterol itself directly induces pyroptosis was its increased concentration in pyroptosis-inducing hyphal cells compared to yeast cells. This was elegantly confirmed through the use of ergosterol-containing and ergosterol-free liposomes in a macrophage pyroptosis model. Only the ergosterol-containing liposomes were able to induce pyroptosis-mediated macrophage lysis. These results strongly suggest that there are mechanisms of ergosterol recognition by macrophages.
One concern about the findings summarized above is related to the fact that ergosterol is a typical plasma membrane component and therefore is not accessible to recognition by macrophage surface receptors. However, a number of C. albicans cell wall mannoproteins and related molecules have been demonstrated to bind and transport extracellular sterols, suggesting external distribution. In fact, ergosterol is a major lipid component of fungal extracellular vesicles, which are the main vehicles of trans-cell wall transport in fungi. The passage of ergosterol-containing vesicles through the cell wall implies that this sterol is a transitory cell wall component, which might facilitate recognition by immune cells. In addition, ergosterol-containing vesicles released extracellularly by C. albicans promote macrophage stimulation. Therefore, a number of regular physiological processes support ergosterol export and consequent participation in immunological events. In this scenario, Koselny and colleagues were the first to demonstrate that fungal ergosterol is immunologically active, which opens several avenues of investigation on how the host responds to sterols and fungal lipids in general.
References
[1] Matsumori, N., Tahara, K., Yamamoto, H., Morooka, A., Doi, M., Oishi, T., & Murata, M. (2009).Direct Interaction between Amphotericin B and Ergosterol in Lipid Bilayers As Revealed by 2H NMR Spectroscopy. Journal of the American Chemical Society, 131(33), 11855-11860.
[2] Hirsch, A. L. (2011). Chapter 6 - Industrial Aspects of Vitamin D. In Vitamin D: Third Edition (pp. 73-93). Academic Press.
[3] Rodrigues, M. L. (2018). The multifunctional fungal ergosterol. mBio, 9(3).
You may like
See also
Lastest Price from Ergosterol manufacturers

US $0.00/kg2025-04-27
- CAS:
- 57-87-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $1.00/KG2025-04-21
- CAS:
- 57-87-4
- Min. Order:
- 1KG
- Purity:
- ≥97%
- Supply Ability:
- 100kg/month