Erdafitinib can be used to treat urothelial cancer
Description
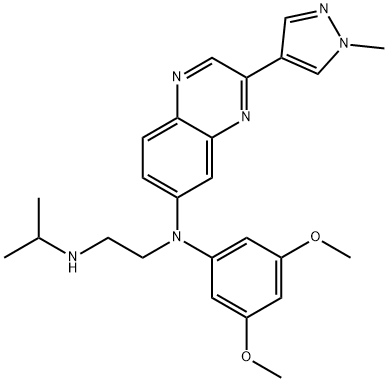
Erdafitinib, also known as JNJ-42756493, is a first-in-class pan-fibroblast growth factor receptor (FGFR) kinase inhibitor discovered by Astex Pharmaceuticals and Janssen. The chemical name of erdafitinib is N-(3,5-Dimethoxy-phenyl)-N'-isopropyl-N-[3-(1-methyl-1 H-pyrazole-4-yl)-quinoxalin-6-yl]-ethane-1,2-diamine[1].
Uses
The drug was approved by the USFDA for the treatment of metastatic urothelial carcinoma with specific FGFR2 and FGFR3 genetic mutations. Based on the results of the phase 2 BLC2001 trial (NCT02365597), in which erdafitinib showed an overall response rate of 40% in metastatic urothelial cancer (U) with FGFR3 mut/fus, it is the first approved targeted therapy in metastatic UC. Urothelial cancer, particularly of the bladder, is the sixth most common cancer, representing 4.5% of all new cancer cases, with an estimated 81,000 new cases and 17,980 deaths in 2020. The drug is also being investigated as a treatment for other cancers, including cholangiocarcinoma, liver cancer, non-small cell lung cancer, prostate cancer, lymphoma, and oesophageal cancer[2].
Biological activity
FGFR genetic mutations play a significant role in the pathogenesis of certain cancers, including urothelial cancer. Erdafitinib binds to and inhibits the enzymatic activity of FGFR1−4, inhibiting phosphorylation and cell signaling. In addition, erdafitinib also binds to Fmsrelated tyrosine kinase 4 (FLT4), the tyrosine−protein kinase c-KIT, and vascular endothelial growth factor receptor 2 (VEGFR-2).
Synthesis method
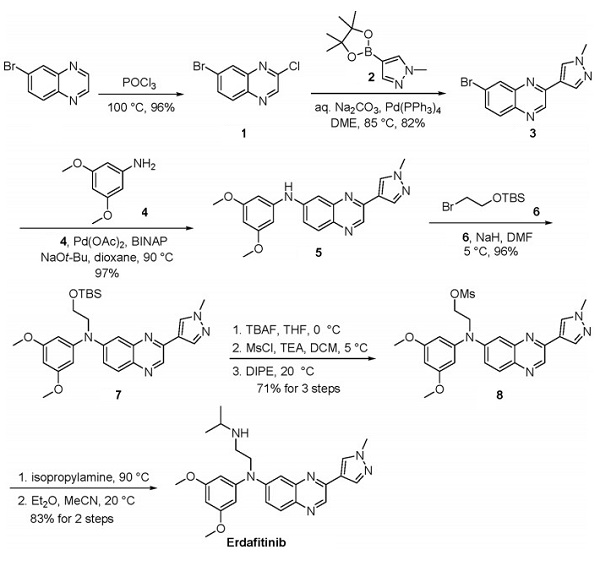
A gram-scale synthesis of erdafitinib has been described that starts with chlorination of 6-bromoquinoxaline [3]. Suzuki coupling of chloride 1 and pyrazole 2 provided bromide 3, which underwent Buchwald−Hartwig coupling with aniline 4. Alkylation of the secondary amine with bromide 6 followed by removal of the silyl ether, mesylation, and recrystallization with DIPE-provided compound 8. Displacement of the mesylate with isopropylamine and subsequent recrystallization from diethyl ether and acetonitrile furnished erdafitinib as the free base in 83% yield.
References
[1] L. Marandino. “Erdafitinib for the treatment of urothelial cancer.” Expert Review of Anticancer Therapy 19 1 (2019): 835–846.
[2] Markham, Anthony. “Erdafitinib: First Global Approval.” Drugs 79 9 (2019): 1017–1021.
[3] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
You may like
See also
Lastest Price from ERDAFITINIB manufacturers

US $0.00-0.00/kg2025-08-22
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1
US $0.00-0.00/kg2025-04-04
- CAS:
- 1346242-81-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton