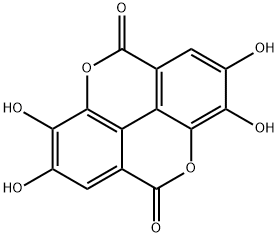
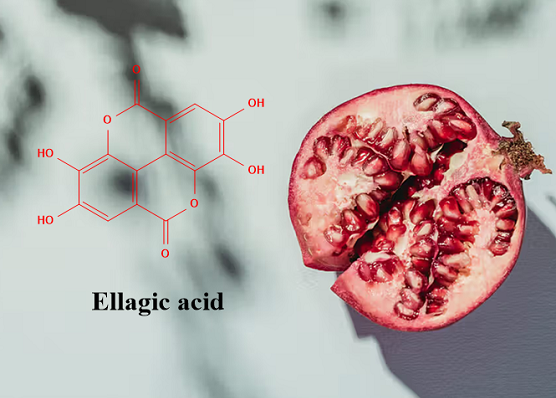
Ellagic acid: Source, Chemical property, and Bioavailability
Introduction
The bioactive compound mainly responsible for the health effects of pomegranate is ellagic acid (EA), as it represents one of the most potent dietary antioxidants. EA is a polyphenol compound derived from ellagitannins (ET), a family of molecules in which hexahydroxydyphenic acid residues are esterified with glucose or quinic acid. Following a hydrolytic process, the hexahydroxydyphenic acid group is released, dehydrates and spontaneously lactonizes, forming EA.
Source
Ellagic acid (EA) was first discovered in 1831 by the French chemist and pharmacist Henri Braconnot, who named it “acide ellagique” from the reverse-read word “galle”. However, the presence of this substance in plants was not clearly understood until the early 20th century, when it was prepared from various plant sources such as oak bark, valonea, pomegranate (Punica granatum L.), divi-divi (Caesalpinia coriaria (Jacq.) Willd.), myrobalan (Terminalia catappa L.), and algarrobilla (Prosopis humilis Hook.). At present, EA is a naturally occurring bioactive and pharmacologically active polyphenolic compound abundant in many taxonomically diverse plant groups, mainly among eudicotyledons.
EA is produced in plants mainly via the hydrolysis of ellagitannins, a widely distributed group of secondary metabolites. Along with free EA, plants capable of synthesizing ellagitannins contain a range of EA derivatives with varying structural complexity, which arise due to methylation, methoxylation, glycosylation, and glucuronidation of its molecule. Ellagitannins, hydrolysable derivatives of EA, can release it in a free form in the human gastrointestinal tract after consuming plant-based foods.
Chemical property
EA is a chromene-dione derivative (2,3,7,8-tetrahydroxy-chromeno [5,4,3-cde]chromene-5,10-dione), encompassing both a hydrophilic moiety with 4 hydroxyl groups and 2 lactone groups, and a planar lipophilic moiety with 2 biphenyl rings. This particular structure has both hydrogen bonding acceptor (lactone) and donor (–OH) sites. Due to the weak acidic nature of its four phenolic groups (pKa1 = 5.6 at 37 °C), around neutral pH, it is mainly deprotonated on positions 8 and 8′. In contrast, above pH 9.6 lactone rings open to give a carboxyl derivative. EA's low oral bioavailability is mostly due to its poor water solubility (9.7 µg/mL), which increases with pH, and the antioxidant action. However, in basic solutions, phenolic compounds lack stability as these molecules, under the ionic form, undergo extensive transformations or are converted to quinones due to oxidation. A stability study on pomegranate fruit peel extract demonstrated that EA content significantly decreases in a few weeks regardless of the pH of the solution due to the hydrolysis of the ester group with hexahydroxydiphenic acid formation, suggesting that EA should not be stored in aqueous medium; this aspect reinforces the need to develop novel systems also for EA stabilization.
Concerning organic solvents, EA is slightly soluble in methanol and DMSO and shows maximum solubility in N-methyl-2-pyrrolidone (NMP), confirming the effect of basic pH on EA dissolution. An analogous trend is also observable in aqueous solutions, where results implied that the solubility of EA depends on the pH values of the media. While EA is almost insoluble in acidic media and distilled water, its water solubility is significantly improved by basic pH. As highlighted further ahead, one of the most exploited vehicles is polyethylene glycol (PEG) 400, as it is endowed with satisfactory biocompatibility and, at the same time, is miscible with both aqueous and organic solvents. EA solubility in oils and surfactants is also provided, which is helpful for developing emulsifying-based techniques.
Bioavailability
It is suggested that the absorption sites of free EA into circulation are either the stomach or small intestine. A small proportion of free EA from the diet is absorbed in the stomach, whereas ellagitannins are resistant to acid hydrolysis and degradation in the stomach. Hydrolysis of ellagitannins and release of EA occurs in the small intestine at a neutral to slightly basic pH, which may allow some freed EA to be absorbed in the small intestine. The uptake process for EA is thought to be via passive diffusion driven by a concentration gradient, with no specific transporters having been identified yet that facilitate EA uptake across the gut epithelium.
Supporting this presumption, the appearance and clearance of EA in plasma are largely dependent on the EA-to-ellagitannin ratio. When a human subject consumed pomegranate juice containing 318 mg ellagitannins and 25 mg free EA, plasma concentrations of EA reached maximum serum concentration (Cmax) = 0.106 μmol/L (32 ng/mL) at time of maximum concentration observed (Tmax) = 1.0 h, which reached a plateau at 4 h. After drinking a single dose of pomegranate juice that contained the same amount of ellagitannins but one-half of the free EA (i.e., 318 mg ellagitannins and 12 mg free EA), plasma concentrations of EA reached Cmax = 0.06 μmol/L (18 ng/mL) at Tmax = 0.98 h, and the elimination half-time was 0.71 h. In a similar study, when human subjects were given 400 mg pomegranate extract that contained 330 mg ellagitannins and 22 mg free EA, the Cmax of EA was found to be 0.11 μmol/L (33 ng/mL) at Tmax = 1 h. In a crossover pharmacokinetic study, human subjects were given either 130 mg punicalagin (the ellagitannin form found in pomegranate) plus 524 mg free EA or 279 mg punicalagin plus 25 mg free EA. Intriguingly, the high dose of free EA did not enhance EA bioavailability compared with the lower dose, suggesting that absorption saturation is reached in the small intestine. The short Tmax suggested that EA was primarily absorbed from the stomach and the upper region of the small intestine. In addition, the rapid elimination of EA from systemic circulation suggests an efficient first-pass metabolism of absorbed EA in the intestine and liver and a weak enterohepatic recirculation. During first-pass metabolism, absorbed EA is converted to methyl esters, dimethyl esters, and glucuronides. These metabolites were detected in human plasma and urine 1–5 h after ingestion of ellagitannins.
References
[1] Javad Sharifi-Rad. “Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential.” IEEE Access 8 1 (2022): 3848084.
[2] G. Zuccari. “Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid.” Applied Sciences-Basel (2020).
You may like
Related articles And Qustion
See also
Lastest Price from Ellagic acid manufacturers

US $1200.00-1100.00/ton2025-10-14
- CAS:
- 476-66-4
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $0.00-0.00/KG2025-04-27
- CAS:
- 476-66-4
- Min. Order:
- 1KG
- Purity:
- 98%,90%,40%
- Supply Ability:
- 2000tons