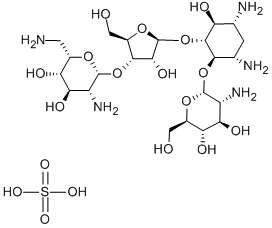
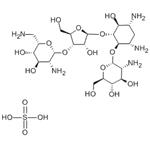
Effect of PAROMOMYCIN SULFATE
The aim of this study was to evaluate the antileishmanial effects of topical liposomal paromomycin sulfate (PM) in Leishmania major-infected BALB/c mice. Liposomes containing 10 or 15% PM (Lip-PM-10 and Lip-PM-15, respectively) were prepared by the fusion method and were characterized for their size and encapsulation efficiency. The penetration of PM from the liposomal PM formulations (LPMFs) through and into skin was evaluated in vitro with Franz diffusion cells fitted with mouse skin at 37°C for 8 h. The in vitro permeation data showed that almost 15% of the LPMFs applied penetrated the mouse skin, and the amount retained in the skin was about 60% for both formulations. The 50% effective doses of Lip-PM-10 and Lip-PM-15 against L. major promastigotes in culture were 65.32 and 59.73 μg/ml, respectively, and those against L. major amastigotes in macrophages were 24.64 and 26.44 μg/ml, respectively. Lip-PM-10 or Lip-PM-15 was used topically twice a day for 4 weeks to treat L. major lesions on BALB/c mice, and the results showed a significantly (P < 0.001) smaller lesion size in the mice in the treated groups than in the mice in the control group, which received either empty liposomes or phosphate-buffered saline (PBS). Eight weeks after the beginning of the treatment, every mouse treated with LPMFs was completely cured. The spleen parasite burden was significantly (P < 0.001) lower in mice treated with Lip-PM-10 or Lip-PM-15 than in mice treated with PBS or control liposomes, but no significant difference was seen between the two groups treated with either Lip-PM-10 or Lip-PM-15. The results suggest that topical liposomal PM may be useful for the treatment of cutaneous leishmaniasis.
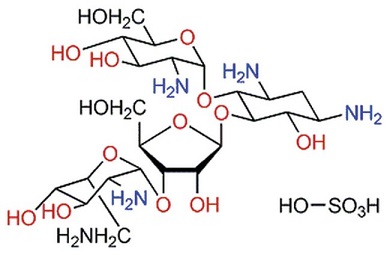
Paromomycin sulfate (PM) was reported to show anti-Leishmania activity in the 1960s, and since then PM showed promising activity against both CL and visceral leishmaniasis (VL) in clinical trials . Recently, the parenteral formulation of PM has been approved for use for the treatment of VL . Nevertheless, the systemic use of PM might cause nephrotoxicity. The conventional topical dosage forms of PM have been tested in clinical trials for their activities against CL and showed promising results, but acceptable efficacy was not always seen. The formidable barrier nature of the stratum corneum (SC) of the skin does not allow the penetration of drugs with high hydrophilicities and molecular weights, like PM. Furthermore, drugs topically applied for the treatment of CL must be able to target the Leishmania parasites within the phagolysosome of the infected macrophages in the deep dermal layer of the skin.
MATERIALS AND METHODS
Animals and parasites.Female BALB/c mice (age, 6 to 8 weeks) were purchased from the Pasteur Institute (Tehran, Iran). The mice were maintained in the Animal House of the Biotechnology Research Center of the Mashhad University of Medical Sciences and were provided with tap water and fed a standard laboratory diet (Khorassan Javane Co., Mashhad, Iran). The animals were housed in a colony room with a 12-h light and 12-h dark cycle at 21°C and had free access to water and food. The animal experiments were carried out according to Ethical Committee Acts of the Mashhad University of Medical Sciences.
The virulence of Leishmania major strain MRHO/IR/75/ER was maintained by passage in BALB/c mice. The amastigotes were isolated from the spleen of an infected mouse and were cultured on NNN (Novy-MacNeal-Nicolle) medium and subcultured in RPMI 1640 (Sigma) containing 10% (vol/vol) heat-inactivated fetal calf serum (FCS), 2 mM glutamine, 100 U/ml of penicillin, and 100 μg/ml of streptomycin sulfate (RPMI-FCS) at 25 ± 1°C.
Chemicals.Soybean phosphatidylcholine (SPC) and cholesterol were obtained from Avanti Polar Lipids (Alabaster, AL). PM, propylparaben (PP), methylparaben (MP), propylene glycol, 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; tissue culture grade), and HEPES were purchased from Sigma; and vitamin E was purchased from Merck (Darmstadt, Germany). 2,4-Dinitro-1-fluorobenzene (DNFB) was purchased from Fluka (Germany).
Preparation of formulations containing PM.Liposomes containing PM were prepared by the fusion method (20). Briefly, the lipid components consisted of SPC (15%), cholesterol (2%), propylene glycol (7%), vitamin E (0.3%), MP (0.1%), and PP (0.02%); these components were melted at about 75°C (lipid melt). HEPES buffer (10 mM, pH 7.0) and PM (10 or 15%) were heated separately and were added up to 100% to the previously heated melted lipids, and the mixture was vigorously vortexed to allow it to cool to room temperature. The final products were then homogenized with an homogenizer (Ultra-Turrax IKA T10; IKA Werke GmbH & Co. KG, Staufen, Germany) for 5 min at 5,000 rpm. The same procedure used for the preparation of liposomal PM described above was used to prepare control empty liposomes, except that the PM was omitted.
Characterization of liposomes.The particle diameter of each sample was measured in triplicate by the use of dynamic light scattering (ZetaSizer Nano-ZS; Malvern Instruments Ltd., Worcestershire, United Kingdom).
To determine the efficiency of incorporation (percent encapsulation), certain amounts of liposomal PM were centrifuged (Universal 320 R; Hettich, Germany) at 14,000 × g for 30 min at 4°C and were subsequently washed three times with HEPES buffer. The supernatant and precipitate were then analyzed for PM by adding 1.5 ml DNFB (150 mM in methanol) to 0.5 ml sample, heating the mixture at 80°C for 45 min, making the volume up to 25 ml with chloroform-tetrahydrofuran-water (25:28.2:0.8), and measuring the absorbance at the maximum wavelength (405 nm) after the upper aqueous phase was discarded. The encapsulation efficiency of PM in liposomes was then calculated indirectly and directly in triplicate, as follows: for the indirect calculation, the percent encapsulation efficiency was equal to [(total amount of PM added − amount of PM recovered in supernatants)/total amount of PM added] × 100, and for the direct calculation, the percent encapsulation efficiency was equal to (amount of PM recovered in liposome precipitate/total amount of PM added) × 100.
Cell diffusion study.Jacketed Franz cells with a receiver volume of 25 ml were used, and every experiment was conducted in triplicate at 37°C. Phosphate buffer of pH 7.4 was used as the receiver medium. A suitable size of full-thickness skin of a BALB/c mouse was cut and mounted in the Franz cell, with the SC side facing upward. The mouse was properly shaved with electric clippers on the day before the experiment. The membranes were initially left in the Franz cells for 30 min in order to facilitate hydration. Subsequently, 1 g of the liposomal PM formulation (LPMF) was deposited onto each membrane surface. A 250-μl aliquot was withdrawn from each receiver solution at 1-h intervals and replaced with the same volume of blank PBS solution. Aliquots of the collected samples were analyzed for their PM content, as described above. The derived concentration values were corrected by using the equation Mt(n) = Vr × Cn + Vs × ΣCm, where Mt(n) is the current cumulative mass of drug transported across the skin at time t, n is the number (times) of sampling, Cn is the current concentration in the receiver medium, ΣCm is the summed total of the previously measured concentrations, Vr is the volume of the receiver medium, and Vs corresponds to the volume of the sample removed for analysis.
For the determination of the amount of liposome retained in the skin, at the end of the experiment, the amount of the formulation remaining on the surface of the membrane was collected and assayed for PM. The amount of PM retained in the skin was then calculated by subtracting the sum of the amount of PM that remained on the surface and the amount of PM that was released (penetrated through the skin) from the whole amount applied.
In vitro promastigote assay.The effects of the formulations on the viability of Leishmania promastigotes were assessed by monitoring MTT metabolism after a 48-h culture period in the presence of the formulations. Parasites were harvested at stationary phase of culture, and 400,000 promastigotes were added to each well of 96-well flat-bottom plates containing different concentrations of the formulations; triplicate wells were used for each concentration. The plates were incubated at 25 ± 1°C for 48 h prior to the addition of MTT (40 μl/well of 5 mg/ml in PBS), and then the plates were incubated in the dark at 37°C for a further 4 h. The formation of formazan was evaluated by adding 50 μl/well 20% sodium dodecyl sulfate and incubating the plates overnight at 37°C, and the relative absorbance was photometrically measured with an enzyme-linked immunosorbent assay reader (Statfax-2100; Awareness Technology) at 545 nm. The relative absorbance was correlated to the number of promastigotes per well by using a standard curve that consisted of the results for different numbers of promastigotes treated with the MTT dye, as explained above. The 50% effective dose (ED50) for each formulation was calculated by the Litchfield-Wilcoxon method with PCS (version 4) software .
In vitro amastigote assay.Cells of the J774 A.1 mouse macrophage cell line (Pasteur Institute) were dispensed at a concentration of 50,000 macrophages/well into eight-well Lab-Tek (Nunc) chamber slides and maintained at 37°C in 5% CO2 for 24 h to allow attachment of the cells. The cells were then infected with L. major promastigotes at a ratio of five promastigotes per macrophage and incubated at 37°C in 5% CO2 for 24 h to allow internalization of the parasites in the cells. The excess amount of promastigotes was removed by gently washing the cells with PBS three times, and the infected cells were incubated for an additional 24 h to allow the establishment of the infection. The cells were then exposed to different concentrations of LPMFs in triplicate for 2 days. The experiment was terminated by methanol fixation of the slides. The slides were then stained with Giemsa and evaluated microscopically to calculate the percentage of infected cells. The ED50 for each formulation was calculated by the Litchfield-Wilcoxon method with PCS (version 4) software.
In vivo experiment.Forty female BALB/c mice (age, 6 to 8 weeks) were inoculated subcutaneously at the base of the tail with 4 × 106 L. major promastigotes harvested at stationary phase. At 4 weeks postinfection, the lesions were measured with calipers in two dimensions, the mean diameters were determined, and the mice were randomly divided into four groups of 10 mice each. No significant differences (P > 0.05) in lesion size were seen among the different groups. The lesions were then treated topically with 50-mg formulations twice a day for 4 weeks. The lesion sizes were measured weekly during treatment and at week 4 after the treatment was stopped.
Quantitative parasite burden.The number of viable L. major parasites in the spleens of the mice was assessed by limiting dilution assay. The mice were killed at 8 and 12 weeks after infection; the spleens were aseptically removed and homogenized in 1 ml RPMI-FCS with a sterile syringe piston. The homogenate was diluted with the same medium in eight serial 10-fold dilutions in each well of flat-bottom 96-well microtiter plates containing a solid layer of rabbit blood agar in triplicate, and the plates were incubated at 25 ± 1°C for 7 days. The positive wells (which contained motile parasites) and the negative wells (which did not contain motile parasites) were identified with an inverted microscope. The data are reported as the calculated mean and standard error of the mean of the last positive well multiplied by the dilution factor.
Statistical analysis.The one-way analysis of variance statistical test was used to assess the significance of the differences among the various groups. In the case of a significant F value, the multiple-comparison Tukey test was used to compare the means of the different treatment groups. Results with P values of <0.05 were considered statistically significant.
RESULTS
Liposome characterization.The liposomes used in this study were in the submicron size range. The zeta average size and the polydispersity of the control liposomes were less than those of the liposomes containing 10 or 15% PM (Lip-PM-10 and Lip-PM-15, respectively); however, no significant difference was seen between Lip-PM-10 and Lip-PM-15.
See also
Lastest Price from PAROMOMYCIN SULFATE manufacturers
US $0.00/kg2025-11-21
- CAS:
- 1263-89-4
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 1263-89-4
- Min. Order:
- 1KG
- Purity:
- ≥675μg/mg; USP
- Supply Ability:
- 500kg