Efavirenz---A first-line agent for HIV-1 infection
Efavirenz is a widely used first-line agent for treatment of HIV-1 infection and is a member of the class of non-nucleoside reverse transcriptase inhibitors. When administered in combination with other antiretroviral drugs, it suppresses HIV-1 replication, improves immunologic function, enhances quality of life, and reduces HIV-related clinical progression and mortality. Efavirenz, in combination with other antiretroviral agents, is widely recommended as a preferred agent for initial treatment of HIV-1 infection in US, UK, European, and World Health Organization (WHO) guidelines . Its main advantages are potency, convenient dosing (once daily), tolerability, and established track record; its chief drawbacks are low genetic barrier to resistance (with cross-resistance to most of the other non-nucleoside agents) if administered as part of an insufficiently potent regimen, neuropsychiatric side-effects, lack of established safety in pregnancy, and its potential contribution to lipodystrophy and metabolic syndrome.
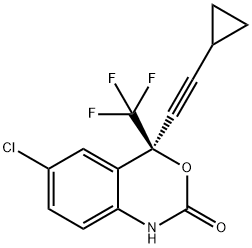
Efavirenz, earlier known as L-743,726 and DMP-266, is a benzoxazinone compound that was developed by DuPont Merck at Merck Research Laboratories and subsequently co-marketed by DuPont Merck Pharmaceuticals. Efavirenz is a selective non-nucleoside inhibitor of the HIV-1 reverse transcriptase and has no activity against cellular alpha, beta or gamma DNA polymerases. The concentration of efavirenz is usually expressed in either ng/ml (or mg/l) or nM; to convert from ng/ml to nM, multiply by 3.16 (i.e. 1 ng/ml is 3.16 nM). Efavirenz is formulated as 50-, 100-, and 200-mg capsules, as 50-, 200-, 300-, and 600-mg tablets and as a solution of 30 mg/ml. Availability of specific formulations varies from country to country.
Efavirenz is currently marketed as Sustivas (Bristol-Myers-Squibb) in the USA, Canada, and some European countries, and as Stocrins (Merck Sharp and Dohme) elsewhere. Generic versions of efavirenz that have been granted tentative approval by the US President’s Emergency Plan for AIDS Relief (PEPFAR) through expedited review by the US Food and Drug Administration (US Food and Drug Administration, 2010) or listed by the WHO Prequalification Program (World Health Organization Prequalification Programme, 2010) are manufactured by Indian pharmaceutical companies (Cipla, Aurobindo, Ranbaxy, Matrix, and Strides Arcolab), and other versions are manufactured (or soon to be so) in Thailand and South Africa. Efavirenz 600 mg is incorporated into a once-daily fixed-dose combination tablet with tenofovir 300 mg plus emtricitabine (FTC) 200 mg (co-produced with Gilead Sciences) and marketed as Atriplas. Generic versions of efavirenz co-packaged with lamivudine 150 mg plus zidovudine 300 mg or lamivudine 150 mg plus stavudine 40 or 30 mg are produced by Indian manufacturers.
MECHANISM OF DRUG ACTION
HIV-1 reverse transcriptase is a heterodimer comprising a p66 subunit (which carries out the reverse transcription and RNA degradation enzymatic functions) and a p51 subunit. Efavirenz binds noncompetitively to a hydrophobic pocket located in the ‘‘palm’’ and ‘‘thumb’’ subdomains of the p66 subunit that is close to but separate from the active site of the enzyme and is formed by complex folding of p66. The reverse transcriptase binding site is common to other non-nucleoside agents nevirapine and delavirdine . Binding of these inhibitors does not prevent the binding of either the nucleotide substrate or the RNA template to p66, but brings about a conformational change in p66 that prevents incorporation of dNTPs into the growing proviral DNA chain. Amino acids involved in resistance to non-nucleoside agents such as K103 and Y181 line the binding site pocket, and alterations in these residues prevent drug binding by steric hindrance. The intrinsic lack of activity of efavirenz, nevirapine, and delavirdine against HIV-2 and HIV-1 type 0 is due to the presence of naturally occurring Y181I and Y181C mutations, respectively . Efavirenz also inhibits late stages of the HIV replicative cycle by enhancing intracellular processing of HIV-1 Gag and Gag-Pol polyproteins, with an associated reduction in viral particle formation, possibly through premature activation of the HIV protease.
You may like
Related articles And Qustion
See also
Lastest Price from Efavirenz manufacturers

US $0.00-0.00/Kg/Bag2025-04-21
- CAS:
- 154598-52-4
- Min. Order:
- 0.1Kg/Bag
- Purity:
- USP
- Supply Ability:
- 20 tons

US $0.00/kg2025-04-02
- CAS:
- 154598-52-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000kg