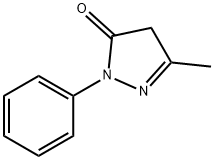
Edaravone: A Breakthrough in Neurological Therapeutics
Introduction
Over the past decades, a multitude of experimental drugs have been shown to delay disease progression in preclinical animal models of amyotrophic lateral sclerosis (ALS) but failed to show efficacy in human clinical trials or are still waiting for approval under Phase I–III trials. A few years ago, an antioxidant drug, edaravone, developed by Mitsubishi Tanabe Pharma was found to be effective in halting ALS progression during early stages. The newly approved drug edaravone is a force multiplier for ALS treatment. This article will make a brief introduction for edaravone.

Figure 1 Characteristics of Edaravone
Clinical Trials1
For edaravone, so far, only one Phase II open‐label trial and two Phase III placebo‐control RCTs have been conducted. In all three trials, the primary outcome evaluated was motor function of patients. Open‐label RCTs suggested that edaravone is safe and effective in ALS. It reduced 3‐nitrotyrosine levels, a marker of oxidative stress. A Phase III confirmatory study (MCI 186‐16: NCT00415519) conducted at 29 RCTs sites in Japan recruited 246 eligible patients, and 206 patients were participated in trial after preobservation period. Changes in ALSFRS‐R mean scores were −5.70 (P = 0.85) in the edaravone‐treated groups compared with −6.35 (P = 0.84) for the placebo group, showing no statistically significant differences between the groups (P = 0.411).
Nevertheless, post‐hoc analysis suggested that edaravone was efficacious in a restricted subgroup (patients with milder symptoms and shorter duration of illness). The definitive Phase III double‐blind, parallel two‐arm, placebo‐control trial conducted in Japan led to the approval of edaravone (MCI186‐19: NCT01492686). Eligibility was restricted to patients with a relatively short disease duration and preserved FVC. The patients met the following four criteria: a) functionality retained most activities of daily living (scores of ≥ 2 points or better on revised ALSFRS‐R), b) normal respiratory function (% FVC ≥ 80%), c) definite or probable ALS based on El Escorial revised criteria, and d) disease duration ≤2 years. The trial enrolled 69 and 68 patients for edaravone (60 mg, IV infusion) and placebo, respectively. The decline in ALSFRS‐R scores from baseline was significantly less in the edaravone‐treated patients as compared with placebo, showing greater functional ability.
Mechanism1
The exact cellular and molecular targets of edaravone are unknown. Edaravone acts as a ROS scavenger and inhibits peroxyl radical (LOO•)‐induced peroxidation systems. One of the most interesting findings suggests that edaravone scavenges H 2 O 2 and protects cells against oxidative stress via upregulation of Peroxiredoxin‐2, downregulation of protein disulfide isomerase A3, and inhibition of apoptosis. The reaction between edaravone and ONOO− is approximately 30‐fold greater than uric acid (physiological scavenger for ONOO−). Edaravone traps •OH and inhibits OH−‐dependent lipid peroxidation or tyrosine nitration induced by ONOO−. Under physiological states, 50% of edaravone is present as an anion form, and electrons released from edaravone anion exert radical scavenging. Afterward, edaravone radicals are generated, react readily with oxygen atoms, and form a peroxyl radical (LOO•) of edaravone, and eventually 2‐oxo‐3-(phenylhydrazone)‐ butanoic acid.
References:
[1] JAISWAL M K. Riluzole and edaravone: A tale of two amyotrophic lateral sclerosis drugs[J]. Medicinal Research Reviews, 2018, 39 2: i-ii, 423-770. DOI:10.1002/med.21528.You may like
Lastest Price from 5-Methyl-2-phenyl-1,2-dihydropyrazol-3-one manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 89-25-8
- Min. Order:
- 100g
- Purity:
- 99%min
- Supply Ability:
- 10KGS

US $10.00/KG2025-04-21
- CAS:
- 89-25-8
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt