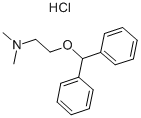
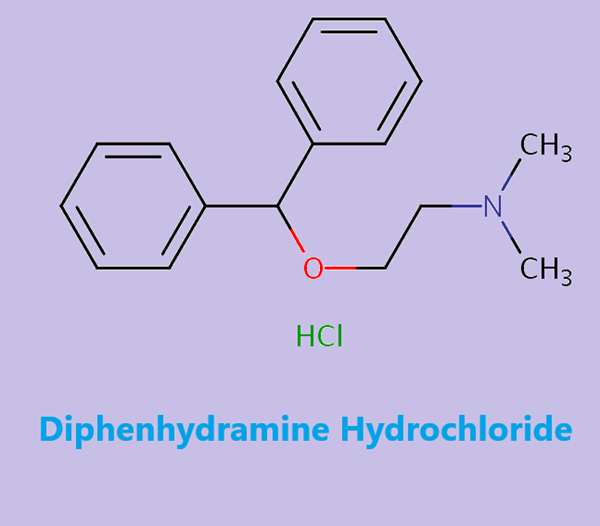
Diphenhydramine Hydrochloride: Overview, Clinical Applications and Side Effects
General Description
Diphenhydramine Hydrochloride has a rich history in treating coughs and allergies. Initially approved in 1948, Diphenhydramine Hydrochloride has evolved to address various conditions such as hay fever, insomnia, and nausea. Despite its effectiveness, concerns about side effects like drowsiness persist. Its diverse pharmacological profile includes antihistamine, antitussive, and sedative properties. Ongoing research explores its potential in managing anxiety and depression. However, users should be cautious of side effects ranging from mild discomfort to severe reactions. Monitoring and reporting any adverse effects are crucial for safe usage.

Figure 1. Diphenhydramine Hydrochloride
Overview
Diphenhydramine Hydrochloride, a compound with a long-standing history in cough remedies, has been utilized for nearly six decades. Initially approved by the Food and Drug Administration (FDA) in 1948 under the brand name Benylin Expectorant, Diphenhydramine Hydrochloride was indicated for the treatment of cough associated with colds and related congestive symptoms. Despite its approval, ongoing correspondence between its manufacturer, Parke, Davis & Co., and the FDA ensued regarding its over-the-counter status and its efficacy versus side effects. Over time, additional FDA approvals broadened its scope of use. In 1985, Diphenhydramine Hydrochloride gained approval for alleviating symptoms of hay fever, allergic rhinitis, and cold-associated sneezing and runny nose. Furthermore, in 1982, it was sanctioned for over-the-counter use as a sleep aid. However, in 1983, an FDA panel assessed its suitability as an antitussive agent. While deeming Diphenhydramine Hydrochloride safe and effective, the FDA Commissioner and the agency disagreed with the panel's recommendation due to concerns regarding excessive drowsiness, attributed to side effects rather than a lack of efficacy. 1
Clinical Applications
Diphenhydramine Hydrochloride, commonly recognized by its brand name Benadryl, serves various clinical applications beyond its initial use as an antihistamine. Primarily a first-generation H1 receptor antihistamine, it finds extensive application in treating seasonal allergies, insect bites, stings, and rashes. Its multifaceted pharmacological profile extends to antiemetic (anti-nausea), antitussive (cough suppression), hypnotic (sleep-inducing), and antiparkinson properties. The sedative effect of Diphenhydramine Hydrochloride stems from its competitive antagonism of histamine H1 receptors in the central nervous system, resulting in its well-known drowsiness side effect. Despite occasional hesitations regarding its sedative nature in allergy therapy, diphenhydramine has found widespread use in over-the-counter sleep aids and nighttime cold medications. Additionally, diphenhydramine is employed in combination with 8-chlorotheophylline to form Dimenhydrinate, primarily utilized for its H1 histamine receptor antagonism in the vestibular system, effectively combating nausea. Furthermore, ongoing research suggests Diphenhydramine Hydrochloride's involvement in various neurotransmitter systems affecting behavior, including dopamine, norepinephrine, serotonin, acetylcholine, and opioid pathways. This exploration has led to investigations into its potential anxiolytic (anxiety-reducing) and antidepressant properties. Diphenhydramine Hydrochloride's versatility in targeting different physiological systems highlights its significance in clinical practice, where it continues to be an essential component in managing a spectrum of conditions, ranging from allergies to sleep disturbances and beyond. 2
Side Effects
Diphenhydramine Hydrochloride, while effective in treating various symptoms, can also cause several side effects, ranging from mild to severe. Common side effects may include drowsiness, dizziness, constipation, stomach upset, blurred vision, or dry mouth, nose, or throat. These effects are usually temporary and may diminish with continued use. To alleviate dry mouth, individuals can try sugarless hard candy or ice chips, sugarless gum, water, or saliva substitutes. It's important to note that if a doctor has prescribed this medication, they have likely assessed that the benefits outweigh the risks for the individual patient. However, some people may experience more serious side effects. These can include changes in mental/mood (such as restlessness or confusion), difficulty urinating, or a fast/irregular heartbeat. In rare cases, Diphenhydramine Hydrochloride can trigger seizures or provoke a severe allergic reaction. Symptoms of a serious allergic reaction may include a rash, itching or swelling, especially of the face, tongue, or throat, severe dizziness, or difficulty breathing. If any of these symptoms occur, it's crucial to seek immediate medical attention. It's essential for individuals to be aware of the potential side effects of Diphenhydramine Hydrochloride and to consult their doctor or pharmacist if they experience any concerning symptoms. Additionally, any adverse effects should be reported to the appropriate regulatory authorities for monitoring and evaluation of drug safety. 3
Reference
1. Björnsdóttir I, Einarson TR, Gudmundsson LS, Einarsdóttir RA. Efficacy of diphenhydramine against cough in humans: a review. Pharm World Sci. 2007; 29(6): 577-583.
2. Diphenhydramine. DrugBank Accession Number: DB01075.
3. Diphenhydramine Hcl - Uses, Side Effects, and More. WebMD.
Related articles And Qustion
See also
Lastest Price from Diphenhydramine Hydrochloride manufacturers

US $0.00-0.00/kg2025-11-17
- CAS:
- 147-24-0
- Min. Order:
- 1kg
- Purity:
- 99%-101%;BP,USP
- Supply Ability:
- 1000KGS

US $0.00/kg2025-05-07
- CAS:
- 147-24-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg