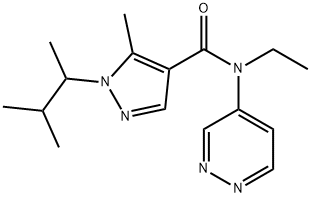
Dimpropyridaz: Mode of action and Synthesis method
Description
Dimpropyridaz (N-Ethyl-5-methyl-1-(3-methylbutan-2-yl)-N-pyridazin-4-ylpyrazole-4-carboxamide) as a new Pyridazine pyrazole carboxamides (PPCs) insecticide developed by BASF Co., Ltd., the mode of action of dimpropytidaz is significantly different from the three insecticides afidopyropen, pymetrozine and flonicamid[1]. While afidopyropen, pymetrozine, and flonicamide exert their insecticidal activities by targeting TRPV channels, dimpropyridaz exerts its insecticidal activity independently of TRPV channels, moreover does not compromise the cellular responses caused by afidopyrogen pymetrozine and flonicamid.
Mode of action
Dimpropyridaz as a proinsecticide is metabolized into an activated metabolite within the target insects, which is targeted on the upstream of the TRPV, decreases the intracellular Ca2+, and blocks the signal transduction of the upstream target site of the TRPV channel. Therefore, by inhibiting the firing of the chordotonal organ and thus disrupting the function of the chordotonal organ, dimpropyridaz exerts its insecticidal activity against insects[2]. Dimpropyridaz is now classified by IRAC as a Pyridazine pyrazole carboxamides (PPCs) insecticide due to its unique mode of action, and it is the first member of this class of insecticides. At present, dimpropyridaz has not been registered in China.
Synthesis method

The synthesis of dimpropyridaz starts from 3-methyl-2-buta- none (160), which is converted into the corresponding hydrazone 161 and then reacted with ethyl 2-acetyl-3-ethoxyacrylate to the β-ketoester 162. This reactive intermediate cyclizes under reductive conditions to the trisubstituted pyrazole derivative 163. Subsequent ester saponication and acid chloride formation lead to 165, which delivers dimpropyridaz by amide formation with N-ethyl-4-pyridazin-amine[3].
References
[1] Meijun Chen, Xusheng Shao*, Zhong Li and Peter Maienfisch*. “Scaffold-Hopping Approach To Identify New Chemotypes of Dimpropyridaz.” Journal of Agricultural and Food Chemistry 70 36 (2022): 11109–11122.
[2] Jiao Shang . “Effects of dimpropyridaz on feeding behavior, locomotivity and biological parameters of Aphis gossypii.” Pesticide Biochemistry and Physiology 197 (2023): Article 105694.
[3] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.