Dimethylamine hydrochloride: applications and toxicology
General Description
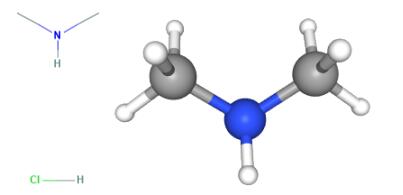
Dimethylamine hydrochloride is a chemical compound that forms when dimethylamine, a secondary amine, reacts with hydrochloric acid. It typically exists in the form of white crystalline solids or powder. Due to its unique properties, dimethylamine hydrochloride has various applications in different industries. In the pharmaceutical industry, N-(2-Chloroethyl) dimethylamine hydrochloride has been studied for wound dressing applications due to its antibacterial properties. In agriculture, dimethylamine hydrochloride can be involved in particle formation reactions with methanesulfonic acid, impacting coastal and agricultural environments. Dimethylamine hydrochloride is also widely used in organic synthesis, including the production of cationic polymers, chelating agents, fabric softeners, and personal care products. However, it's important to note that dimethylamine hydrochloride is toxic to humans and can cause irritation and damage to the body if not handled properly.

Figure 1. Dimethylamine hydrochloride
Applications
Pharmaceutical industry
Dimethylamine hydrochloride, specifically N-(2-Chloroethyl) dimethylamine hydrochloride, has been utilized in the development of a new antibacterial membrane based on quaternized aminochitosan (QAMCS) derivative for advanced wound dressing applications in the pharmaceutical industry. A study aimed to enhance the biological characteristics of chitosan biopolymer, such as biodegradability, biocompatibility, and antibacterial activity. Furthermore, the antibacterial activities of the membranes were assessed against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus cereus. The QAMCS3 membranes demonstrated superior antibacterial activities with maximum inhibition values ranging from 80% to 98%, surpassing the AMCS membrane (57-72%). Additionally, with increasing concentration (from 25 to 250 µg/mL), the antibacterial activities of the membranes significantly improved, as indicated by the minimum inhibition concentration (MIC) results. Moreover, all the membranes displayed excellent biocompatibility and respectable biodegradability, suggesting their potential application as advanced wound dressings. Therefore, the incorporation of dimethylamine hydrochloride in the form of N-(2-Chloroethyl) dimethylamine hydrochloride into the quaternized aminochitosan derivative has shown promise in enhancing the antibacterial properties and overall performance of wound dressings in the pharmaceutical field. 1
Agriculture
Dimethylamine hydrochloride has applications in agriculture relating to the formation of particles resulting from reactions with methanesulfonic acid (MSA). Previous studies have demonstrated that MSA reacts with amines and ammonia, and particle formation is particularly significant in coastal and agricultural areas. In this study, the temperature dependence of particle formation was systematically investigated by reacting MSA with trimethylamine (TMA), dimethylamine (DMA), methylamine (MA), and ammonia under specific conditions. Furthermore, a study highlighted the strong inverse relationship between particle formation and temperature. This indicates that if temperature decreases, such as at higher altitudes or during winter, the decline in particle formation may not be proportional to the concentrations of MSA and amines. These findings provide insights into the complex dynamics of particle formation in coastal and agricultural environments, where Dimethylamine hydrochloride can play a role. 2
Organic synthesis
Dimethylamine hydrochloride is used in a variety of synthetic applications, including the preparation of intermediates for organic synthesis, the quaternization of cellulose for the production of cationic polymers, and the preparation of amine-based chelating agents. Dimethylamine hydrochloride is also used in the textile industry for the preparation of amine-based fabric softeners and in the cosmetics industry for the preparation of hair conditioners and other personal care products. In addition, dimethylamine hydrochloride can be used as a reagent for the derivatization of amino acids and other peptides to enhance their detection by mass spectrometry. Overall, dimethylamine hydrochloride is a valuable reagent for a wide range of synthetic applications due to its ease of preparation and its reactivity as a quaternary ammonium salt. 3
Toxicology
Dimethylamine hydrochloride is a chemical compound that exhibits toxic effects on the human body. It can stimulate the nervous system and cause irritation to the eyes, nose, and throat. Symptoms of high exposure include headache, dizziness, nausea, vomiting, diarrhea, shortness of breath, and coughing. Dimethylamine hydrochloride may also lead to skin irritation and allergic reactions. Moreover, it is corrosive and can damage the digestive and respiratory systems when swallowed or inhaled. Inhalation of dimethylamine hydrochloride may result in chest discomfort, coughing, shortness of breath, and even pneumonia. Prolonged or repeated exposure to high concentrations of dimethylamine hydrochloride can have more severe health consequences. Animal studies suggest potential carcinogenic and mutagenic properties at high doses, though its risk to humans remains uncertain. It is crucial to handle dimethylamine hydrochloride with proper safety precautions, including the use of protective equipment and adequate ventilation in case of exposure. 4
Reference
1. Omer AM, Tamer TM, Khalifa RE, et al.
Formulation and Antibacterial Activity Evaluation of Quaternized
Aminochitosan Membrane for Wound Dressing Applications. Polymers
(Basel), 2021, 13(15):2428.
2. Chen H, Finlayson-Pitts BJ. New Particle Formation from Methanesulfonic Acid and Amines/Ammonia as a Function of Temperature. Environ Sci Technol, 2017, 51(1):243-252.
3. Bi QY, Lin JD, Liu YM, Xie SH, He HY, Cao Y. Partially reduced iridium oxide clusters dispersed on titania as efficient catalysts for facile synthesis of dimethylformamide from CO2, H2 and dimethylamine. Chem Commun (Camb), 2014, 50(65):9138-9140.
4. Huang CC, Kuo HC. Dimethylamine borane neurotoxicity. Environ Health Perspect, 2006, 114(5):A274.
Related articles And Qustion
See also
Lastest Price from Dimethylamine hydrochloride manufacturers

US $0.00-0.00/G2025-04-21
- CAS:
- 506-59-2
- Min. Order:
- 1G
- Purity:
- 99%
- Supply Ability:
- 1 ton/year
US $0.00/kg2025-04-15
- CAS:
- 506-59-2
- Min. Order:
- 200kg
- Purity:
- 98%
- Supply Ability:
- 20 tons