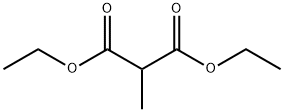
Diethyl Methylmalonate: Chemical Reactant & Enhancer
Diethyl methylmalonate reacts with 2-cyclohexenone under abnormal Michael conditions form 2-(3-oxocyclohexyl)-2-cyclohexenone. It causes the alkylation of poly(chloromethylstyrene) during pahse transfer catalysis. Diethyl methylmalonate was used to investigate the effect of direct supplementation of the medium with di-ester of methylmalonate on erythromycin fermentation in Saccharopolyspora erythraea mutB strain.

An erythromycin process using the diethyl methylmalonate responsive phenotype
The polyketide antibiotic erythromycin A (erythromycin) is produced by Saccharopolyspora erythraea, a gram-positive, mycelial, soil-dwelling actinomycete. This bacterium is widely used for studies of polyketide biosynthesis and strain improvement; and its genome has been completely sequenced and annotated. Although erythromycin has been in use as an antibiotic since the 1950’s, it is now primarily used as an active pharmaceutical ingredient (API) for the production of several semi-synthetic erythromycin derivatives, including azithromycin, clarithromycin, dirithromycin and roxithromycin. Diethyl methylmalonate was chosen as the methylmalonyl-CoA-related compound because it was predicted, due to the ubiquity of cellular esterases, to feed directly into the propionate pathway. It was also chosen because it is used in other industrial processes and is readily available in bulk quantities. Another advantage of diethyl methylmalonate is that it is soluble in the oil component of the erythromycin fermentation medium, and therefore does not lower the pH of the aqueous culture medium as does methylmalonic acid. It is also 2 – 3 times less expensive than methylmalonate, so it is plausible that it could be cost effective to use in large scale fermentations, particularly since low concentrations would be effective. The benefits of using diethyl methylmalonate with the S. erythraea mutB strain are reported here. Also discussed is a new strategy for modifying the classical strain improvement method to take S. erythraea commercial strains beyond their current production plateau.[1]
In order to observe the effect of diethyl methylmalonate on the wild-type strain, shake flask fermentations were performed in triplicate in oil-based broth (OFM1-2x starch) supplemented with it in concentrations ranging from 0 to 25 mM. After five days the cultures were analyzed for erythromycin concentration, pH, viscosity, and presence of non- or mono-glycosylated erythromycin precursors (EB or MEB, respectively). The results show that without supplementation, the wild-type fermentations produced a mean of 790 μg/ml (SD = 32 μg/ml) of erythromycin. With supplementation, the yields went up slightly. Qualitative TLC analysis showed that diethyl methylmalonate caused accumulation of the non-bioactive, non-glycosylated erythromycin precursor, EB. However, it is presumed that not all material was converted to EB because the intensity of the EB spots did not rise beyond the 15 mM level. The pH of the cultures were not affected by supplementation, but viscosity (measured at day five) was improved with supplementation at all levels. Finally, diethyl methylmalonate supplementation reduced the consumption of soybean oil in the medium. The soybean oil layer in the unsupplemented culture was completely depleted by the end of the five day fermentation. The oil layer of the supplemented culture became cloudier, but showed no visible reduction in thickness, indicating that it was not highly metabolized unchanged throughout the fermentation.
In previous studies, genetic experiments indicated that methylmalonyl-CoA was a limiting factor for erythromycin biosynthesis; therefore, in this study, experiments were performed to see whether direct supplementation of the medium with a di-ester of methylmalonate could improve the erythromycin fermentation. When the mutB strain’s diethyl methylmalonate-responsive (Dmr) phenotype was measured in two separate experiments at the 15 mM level it resulted in a 250% increase and a 300% increase in erythromycin production. The 250% increase represented a 55% mole/mole conversion efficiency of diethyl methylmalonate into erythromycin. Starting with the wild type strain, in unsupplemented OFM1-2x starch medium, the results show that diethyl methylmalonate is not converted into erythromycin, indicating that the polyketide precursor pool is not the (sole) limiting factor. Therefore, the model for the wild type strain shows a molar-balanced set of precursor pools leading to erythromycin production. In this case, no one precursor is limiting and therefore all precursor pools need to be raised together to cause a strain improvement. Global mutations that raise all precursor pools simultaneously are the type of mutation that is needed, and are most likely already incorporated into classically-produced commercial strains.
References
[1]Weber JM, Cernota WH, Gonzalez MC, Leach BI, Reeves AR, Wesley RK. An erythromycin process improvement using the diethyl methylmalonate-responsive (Dmr) phenotype of the Saccharopolyspora erythraea mutB strain. Appl Microbiol Biotechnol. 2012 Feb;93(4):1575-83. doi: 10.1007/s00253-011-3650-3. Epub 2011 Nov 4. PMID: 22048617; PMCID: PMC3276693.
You may like
See also
Lastest Price from Diethyl methylmalonate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 609-08-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons

US $0.00-0.00/kg2025-04-04
- CAS:
- 609-08-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton