Diamond:Crystal structure,Uses
Diamond is a solid form of the element carbon, has the highest hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools.
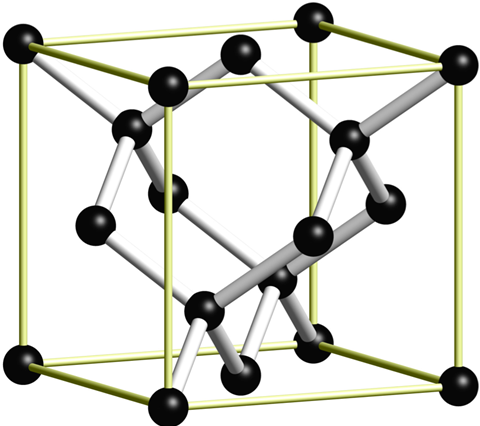
Crystal structure
The most common crystal structure of a diamond is called diamond cubic. It is formed of unit cells stacked together. Although there are 18 atoms in the figure, each corner atom is shared by eight unit cells and each atom in the center of a face is shared by two, so there are a total of eight atoms per unit cell. The length of each side of the unit cell is denoted by a and is 3.567 angstroms.
Uses
The most familiar uses of diamonds today are as gemstones used for adornment and as industrial abrasives for cutting hard materials. The markets for gem-grade and industrial-grade diamonds value diamonds differently.
Most diamonds used as gems are transparent and colorless or nearly so. Colorless or pale blue stones are most valued, but these are rare; most gem diamonds are tinged with yellow. A “fancy” diamond has a distinct body color; red, blue, and green are rare, and orange, violet, yellow, and yellowish green are more common.
Most industrial diamonds are gray or brown and are translucent or opaque, but better-quality industrial stones grade imperceptibly into poor-quality gems. The color of diamonds may be changed by exposure to intense radiation (as released in a nuclear reactor or by a particle accelerator) or by heat treatment.
Natural diamonds
Most natural diamonds have ages between 1 billion and 3.5 billion years. Most were formed at depths between 150 and 250 kilometers (93 and 155 mi) in the Earth's mantle, although a few have come from as deep as 800 kilometers (500 mi). Under high pressure and temperature, carbon-containing fluids dissolved various minerals and replaced them with diamonds.
Synthetic diamonds
Synthetic diamonds can be grown from high-purity carbon under high pressures and temperatures or from hydrocarbon gases by chemical vapor deposition (CVD). Imitation diamonds can also be made out of materials such as cubic zirconia and silicon carbide. Natural, synthetic, and imitation diamonds are most commonly distinguished using optical techniques or thermal conductivity measurements.