Dextrin: Applications and Synthesis
What is Dextrin?
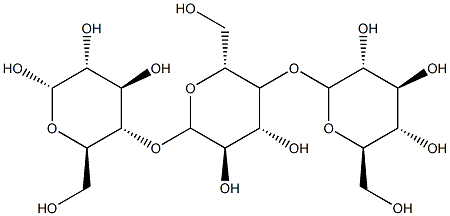
Dextrin is a low molecular weight glucose polymer. It is a generic term for a variety of products obtained by heating starch in the presence of small amounts of water and acid. Dextrins can be made from any starch and are usually classified as white dextrins, yellow dextrins and English gum.

Dextrins have many industrial and food uses. The most common use of dextrin is as an adhesive base for cardboard boxes, envelopes and stamps. It is also used to polish grains and processed fruits, and to decorate ceramics. In the food sector, dextrins enter the food preparation process as thickeners and are used in brewing, baking, fruit juice and cocoa drinks, distilled spirits, confectionery products, and more.
In the pharmaceutical field, dextrin is often used as a pharmaceutical excipient, as a suspending agent, as a tablet binder, and as a diluent for tablets and capsules. It is also used as an ingredient in tablet icing and as a binder and hardener for surgical dressings.
Synthesis of Dextrin
Dextrin is mainly prepared from starch, but can also be chemically synthesised. Commonly used starches include: corn, potato or tapioca starch.
Dextrins are made from corn starch, which is roasted and then hydrolysed by amylase, an enzyme that digests starch ingested as food. Indigestible dextrin is a water-soluble dietary fibre that is extracted and prepared from the indigestible components of the paste.
Polyethylene glycol (PEG) was further applied for fractionating dextrin prepared from cassava starch. The initial dextrin concentration and pH of the dextrin solutions were crucially considered in this study with the average molecular-weight dispersity (DMa) as the index. The results showed that the initial dextrin concentration significantly affected the mass fraction and the molecular weight distribution of each dextrin fraction obtained from gradient PEG precipitation. However, the initial dextrin concentration, which ranged from 0.9% to 3.6%, did not affect the DMa of the dextrin fractions. Furthermore, the DMa of the fractions obtained at pHs 4.00, 4.96, 6.00, 6.92, 7.99, 8.96, and 9.91, was 1.364, 1.341, 1.305, 1.286, 1.273, 1.311, and 1.404, respectively, while the dispersity of the parent dextrin was 2.052. These results suggest that the preparative approach, gradient PEG precipitation, is applicable in acidic, neutral, and alkaline environments, and that a weakly alkaline environment is optimal for dextrin fractionation.
Production of Dextrin by Steam Explosion from Starch Extracted from Oil Palm Stem Waste
This research aimed to modify a starch obtained from palm oil production waste to produce dextrin through steam explosion (SE). The SE process was performed at different temperatures (120, 130, 140, 150, and 160 °C), and the physicochemical characteristics of the dextrin products – including the dextrin solubility, moisture content, and ash content – were analyzed. Besides, SEM image, XRD profile, FTIR profile, DSC profile, dextrose equivalent (DE) and molecular weights (MWs) were examined. The results showed that the SE process at 120 °C gave the best characteristics of dextrin, which was comparable to the commercial dextrin (CD). This dextrin had the best solubility among other products. The SEM image showed that the starch completely hydrolyzed into dextrin after SE treatment, and the dextrin crystal was mostly in irregular form. The XRD profile of the dextrin exhibited the same diffractogram patterns with a peak at 2-theta = 19.4. The FTIR profiles of the products exhibited main peaks at 1148, 1077, 994, and 928 cm–1, which were almost similar to the CD. The transition enthalpy (ΔH) of the dextrin (8.59 J/g) was greater than that of the CD (6.18 J/g), exhibiting a better hydrolysis process on the starch. Among the temperature treatments, the highest DE value was obtained for the SE treatment at 120 °C (10.33), which was still less than that of CD (18.46). The MW of the product was 102000 g/mol, which was higher than that of CD (44000 g/mol).
References:
[1] XIUTING HU . Preparative fractionation of dextrin by polyethylene glycol: Effects of initial dextrin concentration and pH[J]. Journal of Chromatography A, 2017. DOI:10.1016/j.chroma.2017.11.018.
[2] SYARIFAH YUSRA. Production of Dextrin by Steam Explosion from Starch Extracted from Oil Palm Stem Waste[J]. Philippine Journal of Science, 2022. DOI:10.56899/151.03.16.
[3] MARIA MOLINOS. Development of a Hybrid Dextrin Hydrogel Encapsulating Dextrin Nanogel As Protein Delivery System[J]. Biomacromolecules, 2012. DOI:10.1021/bm2015834.
You may like
Related articles And Qustion
See also
Lastest Price from Dextrin manufacturers

US $0.00/kg2025-06-03
- CAS:
- 9004-53-9
- Min. Order:
- 25kg
- Purity:
- N/A
- Supply Ability:
- 20 TONS

US $0.00/kg2025-04-27
- CAS:
- 9004-53-9
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg