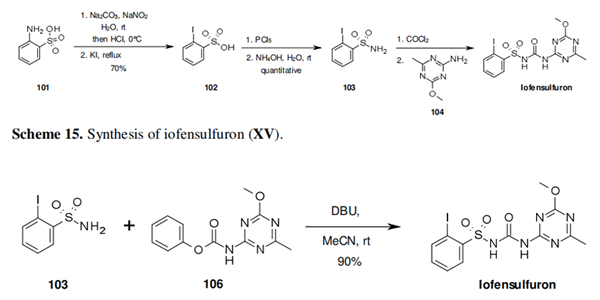
Cyclopyrimorate: Synthesis, Application and Mode of action
Synthesis of Cyclopyrimorate
Cyclopyrimorate is synthesised from two intermediate compounds by coupling reactions, among others. The specific synthesis steps are as follows:
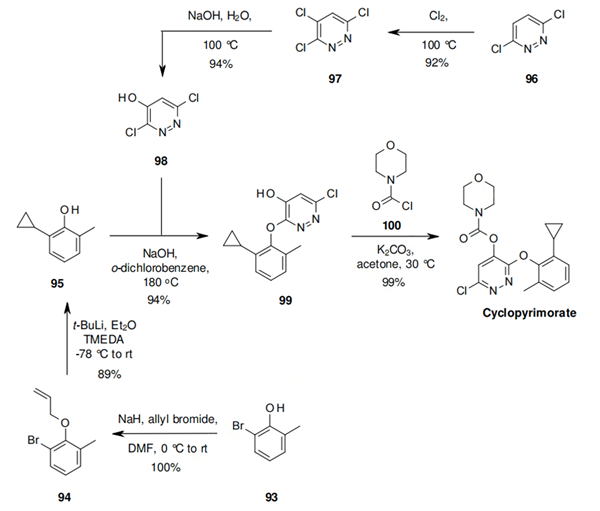
Alkylation of 93 under standard conditions leads to 94, which undergoes an allowed 6-exo-dig cyclisation after anion generation, to the key cyclopropyl intermediate 95. The other key intermediate 98 can be prepared in excellent overall yield by chlorination of 96, followed by selective hydrolysis. The coupling of 98 with 95 was optimized to give 99 in excellent yield and with high chemoselectivity, in a procedure that involves preparing anhydrous NaOH in o-dichlorobenzene by azeotropic removal of water, and then adding 98 in t-BuOH, with continued azeotropic removal of t-BuOH during the reaction. The synthesis is completed by acylation of 99 with 100 under standard conditions.
Application of Cyclopyrimorate
Cyclopyrimorate is a new pyridazine selective broad-spectrum rice herbicide discovered by Daiichi Sankyo (now Mitsui Chemicals Agro). Cyclopyrimorate, a highly effective bleaching herbicide for weed control in rice fields, targets homogentisate solanesyltransferase (HST). HST is a downstream enzyme of 4-hydroxyphenylpyruvate dioxygenase in the plastoquinone (PQ) biosynthesis pathway. Cyclopyrimorate shows synergistic effects with 4-HPPD inhibitors, such as pyrazolynate.
Mode of action
The mode of action of cyclopyrimorate is antagonistic inhibition of auxin-induced growth. Cyclopyrimorate caused bleaching symptoms in Arabidopsis thaliana similar to those caused by existing carotenoid biosynthesis inhibitors, mesotrione and norflurazon. However, cyclopyrimorate treatment resulted in significant accumulation of homogentisate and a reduction in the level of plastoquinone. A metabolite of cyclopyrimorate, des-morpholinocarbonyl cyclopyrimorate (DMC), was detected in plants. These data suggested that cyclopyrimorate and/or DMC inhibit homogentisate solanesyltransferase (HST), a downstream enzyme of 4-hydroxyphenylpyruvate dioxygenase in the plastoquinone biosynthesis pathway. In vitro assays showed that A. thaliana HST was strongly inhibited by DMC and weakly by cyclopyrimorate, whereas other commercial bleaching herbicides did not inhibit HST. DMC derivatives showed a positive correlation between HST inhibition and in vivo bleaching activities. These results indicate that the target site of cyclopyrimorate and DMC is HST, a novel target site of commercial herbicides.
References:
[1] MAMIKO SHINO. Action mechanism of bleaching herbicide cyclopyrimorate, a novel homogentisate solanesyltransferase inhibitor.[J]. Journal of Pesticide Science, 2018, 43 4: 233-239. DOI:10.1584/jpestics.D18-008.
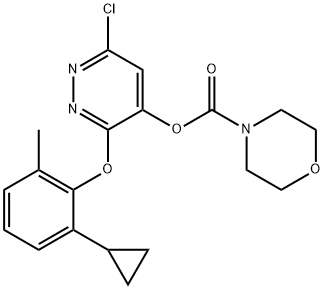 4-Morpholinecarboxylic acid, 6-chloro-3-(2-cyclopropyl-6-methylphenoxy)-4-pyridazinyl ester
4-Morpholinecarboxylic acid, 6-chloro-3-(2-cyclopropyl-6-methylphenoxy)-4-pyridazinyl ester 499231-24-2