Synthetic route to the herbicide Iofensulfuron
Synthesis of Iofensulfuron
Iofensulfuron is synthesised using 2-aminobenzenesulfonic acid as a raw material by chemical reaction. The specific synthesis steps are as follows:
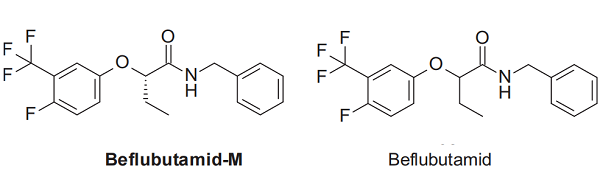
The first step in the synthesis of iofensulfuron involves a Sandmeyer iodination of 2-aminobenzenesulfonic acid (101) yielding 2-iodobenzenesulfonic acid (102). This can be readily transformed into its sulfonamide analogue 103 via sequential treatment with phosphorous pentachloride and ammonium hydroxide. It is likely that the last step of the manufacturing route of iofensulfuron would involve reaction of sulfonamide 103 with phosgene, and subsequent reaction with aminotriazine 104, as depicted in scheme 15. However, the only synthesis of iofensulfuron reported by Bayer CropScience involves the reaction of sulfonamide 103 with carbamate 106 (Scheme 16).
Introduction of Iofensulfuron
Iofensulfuron received a common name from ISO in 2011, and it is known that Bayer CropScience plans to commercialize this compound as its sodium salt. Iofensulfuron is a herbicide sulfonylurea and, as other active ingredients from this chemistry class, an inhibitor of ALS, an enzyme common to the biosynthesis of branched chain amino acids.